US8623288B1 - Apparatus and methods for high density nanowire growth - Google Patents
Apparatus and methods for high density nanowire growth Download PDFInfo
- Publication number
- US8623288B1 US8623288B1 US12/824,485 US82448510A US8623288B1 US 8623288 B1 US8623288 B1 US 8623288B1 US 82448510 A US82448510 A US 82448510A US 8623288 B1 US8623288 B1 US 8623288B1
- Authority
- US
- United States
- Prior art keywords
- cartridge assembly
- nanowire growth
- nanowires
- support layers
- sheet
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active, expires
Links
- 239000002070 nanowire Substances 0.000 title claims abstract description 186
- 238000000034 method Methods 0.000 title claims abstract description 73
- 239000003054 catalyst Substances 0.000 claims description 67
- 239000000463 material Substances 0.000 claims description 64
- 239000002243 precursor Substances 0.000 claims description 45
- 239000010931 gold Substances 0.000 claims description 35
- 125000006850 spacer group Chemical group 0.000 claims description 30
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 claims description 27
- 229910052737 gold Inorganic materials 0.000 claims description 26
- 239000000084 colloidal system Substances 0.000 claims description 24
- 229910052751 metal Inorganic materials 0.000 claims description 21
- 239000002184 metal Substances 0.000 claims description 21
- 238000004519 manufacturing process Methods 0.000 claims description 14
- 230000000712 assembly Effects 0.000 abstract description 3
- 238000000429 assembly Methods 0.000 abstract description 3
- 239000010410 layer Substances 0.000 description 86
- 239000007789 gas Substances 0.000 description 74
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 48
- 239000011888 foil Substances 0.000 description 27
- 239000000758 substrate Substances 0.000 description 18
- 238000003306 harvesting Methods 0.000 description 15
- 238000005229 chemical vapour deposition Methods 0.000 description 14
- 239000002245 particle Substances 0.000 description 14
- 239000000243 solution Substances 0.000 description 14
- 229910052782 aluminium Inorganic materials 0.000 description 12
- 239000000203 mixture Substances 0.000 description 12
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 11
- PNEYBMLMFCGWSK-UHFFFAOYSA-N aluminium oxide Inorganic materials [O-2].[O-2].[O-2].[Al+3].[Al+3] PNEYBMLMFCGWSK-UHFFFAOYSA-N 0.000 description 11
- 239000010408 film Substances 0.000 description 11
- BLRPTPMANUNPDV-UHFFFAOYSA-N Silane Chemical compound [SiH4] BLRPTPMANUNPDV-UHFFFAOYSA-N 0.000 description 10
- 239000002019 doping agent Substances 0.000 description 10
- 239000002105 nanoparticle Substances 0.000 description 10
- 239000002071 nanotube Substances 0.000 description 10
- 238000000527 sonication Methods 0.000 description 10
- 229910052710 silicon Inorganic materials 0.000 description 9
- 229910003910 SiCl4 Inorganic materials 0.000 description 8
- 238000001035 drying Methods 0.000 description 8
- 238000004049 embossing Methods 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- FDNAPBUWERUEDA-UHFFFAOYSA-N silicon tetrachloride Chemical compound Cl[Si](Cl)(Cl)Cl FDNAPBUWERUEDA-UHFFFAOYSA-N 0.000 description 8
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 7
- 238000001878 scanning electron micrograph Methods 0.000 description 7
- 239000004065 semiconductor Substances 0.000 description 6
- 238000011282 treatment Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 238000001816 cooling Methods 0.000 description 5
- 230000005496 eutectics Effects 0.000 description 5
- 238000010438 heat treatment Methods 0.000 description 5
- 238000011068 loading method Methods 0.000 description 5
- 230000000737 periodic effect Effects 0.000 description 5
- 229910000077 silane Inorganic materials 0.000 description 5
- 229910007264 Si2H6 Inorganic materials 0.000 description 4
- 125000004429 atom Chemical group 0.000 description 4
- PZPGRFITIJYNEJ-UHFFFAOYSA-N disilane Chemical compound [SiH3][SiH3] PZPGRFITIJYNEJ-UHFFFAOYSA-N 0.000 description 4
- 229910052711 selenium Inorganic materials 0.000 description 4
- 229910052714 tellurium Inorganic materials 0.000 description 4
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 3
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 3
- 239000007900 aqueous suspension Substances 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 238000004140 cleaning Methods 0.000 description 3
- 238000000354 decomposition reaction Methods 0.000 description 3
- 238000001914 filtration Methods 0.000 description 3
- 229910052733 gallium Inorganic materials 0.000 description 3
- -1 gold Chemical class 0.000 description 3
- 229910052738 indium Inorganic materials 0.000 description 3
- XEEYBQQBJWHFJM-UHFFFAOYSA-N iron Substances [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000000873 masking effect Effects 0.000 description 3
- 150000002739 metals Chemical class 0.000 description 3
- 239000002074 nanoribbon Substances 0.000 description 3
- 239000002073 nanorod Substances 0.000 description 3
- 238000000059 patterning Methods 0.000 description 3
- 239000010703 silicon Substances 0.000 description 3
- 239000010935 stainless steel Substances 0.000 description 3
- 229910001220 stainless steel Inorganic materials 0.000 description 3
- 229910052717 sulfur Inorganic materials 0.000 description 3
- 229910052718 tin Inorganic materials 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 2
- KDLHZDBZIXYQEI-UHFFFAOYSA-N Palladium Chemical compound [Pd] KDLHZDBZIXYQEI-UHFFFAOYSA-N 0.000 description 2
- 108010039918 Polylysine Proteins 0.000 description 2
- 229910045601 alloy Inorganic materials 0.000 description 2
- 239000000956 alloy Substances 0.000 description 2
- VXAUWWUXCIMFIM-UHFFFAOYSA-M aluminum;oxygen(2-);hydroxide Chemical compound [OH-].[O-2].[Al+3] VXAUWWUXCIMFIM-UHFFFAOYSA-M 0.000 description 2
- 238000000231 atomic layer deposition Methods 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 229910052796 boron Inorganic materials 0.000 description 2
- 229910052799 carbon Inorganic materials 0.000 description 2
- 239000002041 carbon nanotube Substances 0.000 description 2
- 229910021393 carbon nanotube Inorganic materials 0.000 description 2
- 125000001309 chloro group Chemical group Cl* 0.000 description 2
- 238000000576 coating method Methods 0.000 description 2
- 238000004891 communication Methods 0.000 description 2
- 229910052802 copper Inorganic materials 0.000 description 2
- 239000010949 copper Substances 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 239000000446 fuel Substances 0.000 description 2
- 229910052732 germanium Inorganic materials 0.000 description 2
- 239000011521 glass Substances 0.000 description 2
- 230000033444 hydroxylation Effects 0.000 description 2
- 238000005805 hydroxylation reaction Methods 0.000 description 2
- 229910052742 iron Inorganic materials 0.000 description 2
- 239000000155 melt Substances 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 239000002086 nanomaterial Substances 0.000 description 2
- 230000003647 oxidation Effects 0.000 description 2
- 238000007254 oxidation reaction Methods 0.000 description 2
- 229910052698 phosphorus Inorganic materials 0.000 description 2
- XHXFXVLFKHQFAL-UHFFFAOYSA-N phosphoryl trichloride Chemical compound ClP(Cl)(Cl)=O XHXFXVLFKHQFAL-UHFFFAOYSA-N 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000656 polylysine Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 229910010271 silicon carbide Inorganic materials 0.000 description 2
- 229910052709 silver Inorganic materials 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 238000001308 synthesis method Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- YBNMDCCMCLUHBL-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 4-pyren-1-ylbutanoate Chemical compound C=1C=C(C2=C34)C=CC3=CC=CC4=CC=C2C=1CCCC(=O)ON1C(=O)CCC1=O YBNMDCCMCLUHBL-UHFFFAOYSA-N 0.000 description 1
- 229910017115 AlSb Inorganic materials 0.000 description 1
- 229910015849 BeSiN2 Inorganic materials 0.000 description 1
- 229910015894 BeTe Inorganic materials 0.000 description 1
- 229910004608 CdSnAs2 Inorganic materials 0.000 description 1
- 229910004613 CdTe Inorganic materials 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- RYGMFSIKBFXOCR-UHFFFAOYSA-N Copper Chemical compound [Cu] RYGMFSIKBFXOCR-UHFFFAOYSA-N 0.000 description 1
- 229910021589 Copper(I) bromide Inorganic materials 0.000 description 1
- 229910021591 Copper(I) chloride Inorganic materials 0.000 description 1
- 229910021593 Copper(I) fluoride Inorganic materials 0.000 description 1
- 229910021595 Copper(I) iodide Inorganic materials 0.000 description 1
- VMQMZMRVKUZKQL-UHFFFAOYSA-N Cu+ Chemical compound [Cu+] VMQMZMRVKUZKQL-UHFFFAOYSA-N 0.000 description 1
- 229910016518 CuGeP3 Inorganic materials 0.000 description 1
- 229910016351 CuSi2P3 Inorganic materials 0.000 description 1
- 229910002601 GaN Inorganic materials 0.000 description 1
- 229910005540 GaP Inorganic materials 0.000 description 1
- 229910005542 GaSb Inorganic materials 0.000 description 1
- GYHNNYVSQQEPJS-UHFFFAOYSA-N Gallium Chemical compound [Ga] GYHNNYVSQQEPJS-UHFFFAOYSA-N 0.000 description 1
- 229910001218 Gallium arsenide Inorganic materials 0.000 description 1
- 229910005987 Ge3N4 Inorganic materials 0.000 description 1
- 229910005829 GeS Inorganic materials 0.000 description 1
- 229910005866 GeSe Inorganic materials 0.000 description 1
- 229910005900 GeTe Inorganic materials 0.000 description 1
- 229910005939 Ge—Sn Inorganic materials 0.000 description 1
- 229910004042 HAuCl4 Inorganic materials 0.000 description 1
- 229910004262 HgTe Inorganic materials 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229910000673 Indium arsenide Inorganic materials 0.000 description 1
- GPXJNWSHGFTCBW-UHFFFAOYSA-N Indium phosphide Chemical compound [In]#P GPXJNWSHGFTCBW-UHFFFAOYSA-N 0.000 description 1
- ZOKXTWBITQBERF-UHFFFAOYSA-N Molybdenum Chemical compound [Mo] ZOKXTWBITQBERF-UHFFFAOYSA-N 0.000 description 1
- 229910019213 POCl3 Inorganic materials 0.000 description 1
- 229910002665 PbTe Inorganic materials 0.000 description 1
- KJTLSVCANCCWHF-UHFFFAOYSA-N Ruthenium Chemical compound [Ru] KJTLSVCANCCWHF-UHFFFAOYSA-N 0.000 description 1
- 229910018540 Si C Inorganic materials 0.000 description 1
- 229910008045 Si-Si Inorganic materials 0.000 description 1
- 229910008355 Si-Sn Inorganic materials 0.000 description 1
- 229910052581 Si3N4 Inorganic materials 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 229910021607 Silver chloride Inorganic materials 0.000 description 1
- 229910021608 Silver(I) fluoride Inorganic materials 0.000 description 1
- 229910008310 Si—Ge Inorganic materials 0.000 description 1
- 229910006411 Si—Si Inorganic materials 0.000 description 1
- 229910006453 Si—Sn Inorganic materials 0.000 description 1
- 229910005642 SnTe Inorganic materials 0.000 description 1
- ATJFFYVFTNAWJD-UHFFFAOYSA-N Tin Chemical compound [Sn] ATJFFYVFTNAWJD-UHFFFAOYSA-N 0.000 description 1
- RTAQQCXQSZGOHL-UHFFFAOYSA-N Titanium Chemical compound [Ti] RTAQQCXQSZGOHL-UHFFFAOYSA-N 0.000 description 1
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 1
- QCWXUUIWCKQGHC-UHFFFAOYSA-N Zirconium Chemical compound [Zr] QCWXUUIWCKQGHC-UHFFFAOYSA-N 0.000 description 1
- 229910007475 ZnGeP2 Inorganic materials 0.000 description 1
- 229910007707 ZnSnSb2 Inorganic materials 0.000 description 1
- 229910007709 ZnTe Inorganic materials 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000012790 adhesive layer Substances 0.000 description 1
- RREGISFBPQOLTM-UHFFFAOYSA-N alumane;trihydrate Chemical compound O.O.O.[AlH3] RREGISFBPQOLTM-UHFFFAOYSA-N 0.000 description 1
- 229910052787 antimony Inorganic materials 0.000 description 1
- 229910052785 arsenic Inorganic materials 0.000 description 1
- 239000013590 bulk material Substances 0.000 description 1
- 229910052793 cadmium Inorganic materials 0.000 description 1
- UHYPYGJEEGLRJD-UHFFFAOYSA-N cadmium(2+);selenium(2-) Chemical compound [Se-2].[Cd+2] UHYPYGJEEGLRJD-UHFFFAOYSA-N 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 239000012159 carrier gas Substances 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- OXBLHERUFWYNTN-UHFFFAOYSA-M copper(I) chloride Chemical compound [Cu]Cl OXBLHERUFWYNTN-UHFFFAOYSA-M 0.000 description 1
- 229910052593 corundum Inorganic materials 0.000 description 1
- 229910021419 crystalline silicon Inorganic materials 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- 238000003618 dip coating Methods 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 239000001307 helium Substances 0.000 description 1
- 229910052734 helium Inorganic materials 0.000 description 1
- SWQJXJOGLNCZEY-UHFFFAOYSA-N helium atom Chemical compound [He] SWQJXJOGLNCZEY-UHFFFAOYSA-N 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 238000007654 immersion Methods 0.000 description 1
- WPYVAWXEWQSOGY-UHFFFAOYSA-N indium antimonide Chemical compound [Sb]#[In] WPYVAWXEWQSOGY-UHFFFAOYSA-N 0.000 description 1
- RPQDHPTXJYYUPQ-UHFFFAOYSA-N indium arsenide Chemical compound [In]#[As] RPQDHPTXJYYUPQ-UHFFFAOYSA-N 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000009413 insulation Methods 0.000 description 1
- 229910052741 iridium Inorganic materials 0.000 description 1
- GKOZUEZYRPOHIO-UHFFFAOYSA-N iridium atom Chemical compound [Ir] GKOZUEZYRPOHIO-UHFFFAOYSA-N 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- WPBNNNQJVZRUHP-UHFFFAOYSA-L manganese(2+);methyl n-[[2-(methoxycarbonylcarbamothioylamino)phenyl]carbamothioyl]carbamate;n-[2-(sulfidocarbothioylamino)ethyl]carbamodithioate Chemical compound [Mn+2].[S-]C(=S)NCCNC([S-])=S.COC(=O)NC(=S)NC1=CC=CC=C1NC(=S)NC(=O)OC WPBNNNQJVZRUHP-UHFFFAOYSA-L 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 238000001465 metallisation Methods 0.000 description 1
- 238000001000 micrograph Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 229910003465 moissanite Inorganic materials 0.000 description 1
- 229910052750 molybdenum Inorganic materials 0.000 description 1
- 239000011733 molybdenum Substances 0.000 description 1
- 239000002159 nanocrystal Substances 0.000 description 1
- 229910052759 nickel Inorganic materials 0.000 description 1
- 229910052758 niobium Inorganic materials 0.000 description 1
- 239000010955 niobium Substances 0.000 description 1
- GUCVJGMIXFAOAE-UHFFFAOYSA-N niobium atom Chemical compound [Nb] GUCVJGMIXFAOAE-UHFFFAOYSA-N 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 229920000620 organic polymer Polymers 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- 238000000643 oven drying Methods 0.000 description 1
- 229910052763 palladium Inorganic materials 0.000 description 1
- SLIUAWYAILUBJU-UHFFFAOYSA-N pentacene Chemical compound C1=CC=CC2=CC3=CC4=CC5=CC=CC=C5C=C4C=C3C=C21 SLIUAWYAILUBJU-UHFFFAOYSA-N 0.000 description 1
- 238000010587 phase diagram Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229910052703 rhodium Inorganic materials 0.000 description 1
- 239000010948 rhodium Substances 0.000 description 1
- MHOVAHRLVXNVSD-UHFFFAOYSA-N rhodium atom Chemical compound [Rh] MHOVAHRLVXNVSD-UHFFFAOYSA-N 0.000 description 1
- 229910052707 ruthenium Inorganic materials 0.000 description 1
- 238000004626 scanning electron microscopy Methods 0.000 description 1
- SBIBMFFZSBJNJF-UHFFFAOYSA-N selenium;zinc Chemical compound [Se]=[Zn] SBIBMFFZSBJNJF-UHFFFAOYSA-N 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- ADZWSOLPGZMUMY-UHFFFAOYSA-M silver bromide Chemical compound [Ag]Br ADZWSOLPGZMUMY-UHFFFAOYSA-M 0.000 description 1
- HKZLPVFGJNLROG-UHFFFAOYSA-M silver monochloride Chemical compound [Cl-].[Ag+] HKZLPVFGJNLROG-UHFFFAOYSA-M 0.000 description 1
- REYHXKZHIMGNSE-UHFFFAOYSA-M silver monofluoride Chemical compound [F-].[Ag+] REYHXKZHIMGNSE-UHFFFAOYSA-M 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 238000002791 soaking Methods 0.000 description 1
- MBEGFNBBAVRKLK-UHFFFAOYSA-N sodium;iminomethylideneazanide Chemical compound [Na+].[NH-]C#N MBEGFNBBAVRKLK-UHFFFAOYSA-N 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000005507 spraying Methods 0.000 description 1
- 238000004544 sputter deposition Methods 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 229910052715 tantalum Inorganic materials 0.000 description 1
- GUVRBAGPIYLISA-UHFFFAOYSA-N tantalum atom Chemical compound [Ta] GUVRBAGPIYLISA-UHFFFAOYSA-N 0.000 description 1
- OCGWQDWYSQAFTO-UHFFFAOYSA-N tellanylidenelead Chemical compound [Pb]=[Te] OCGWQDWYSQAFTO-UHFFFAOYSA-N 0.000 description 1
- 229910052716 thallium Inorganic materials 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 229910052719 titanium Inorganic materials 0.000 description 1
- 239000010936 titanium Substances 0.000 description 1
- 229910052723 transition metal Inorganic materials 0.000 description 1
- 229910000314 transition metal oxide Inorganic materials 0.000 description 1
- 150000003624 transition metals Chemical class 0.000 description 1
- WFKWXMTUELFFGS-UHFFFAOYSA-N tungsten Chemical compound [W] WFKWXMTUELFFGS-UHFFFAOYSA-N 0.000 description 1
- 229910052721 tungsten Inorganic materials 0.000 description 1
- 239000010937 tungsten Substances 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 229910052720 vanadium Inorganic materials 0.000 description 1
- LEONUFNNVUYDNQ-UHFFFAOYSA-N vanadium atom Chemical compound [V] LEONUFNNVUYDNQ-UHFFFAOYSA-N 0.000 description 1
- 235000012431 wafers Nutrition 0.000 description 1
- 229910001845 yogo sapphire Inorganic materials 0.000 description 1
- 229910052725 zinc Inorganic materials 0.000 description 1
- 229910052726 zirconium Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B29/00—Single crystals or homogeneous polycrystalline material with defined structure characterised by the material or by their shape
- C30B29/02—Elements
- C30B29/06—Silicon
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/0053—Details of the reactor
- B01J19/006—Baffles
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/24—Stationary reactors without moving elements inside
- B01J19/2415—Tubular reactors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/24—Stationary reactors without moving elements inside
- B01J19/248—Reactors comprising multiple separated flow channels
- B01J19/2495—Net-type reactors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/32—Packing elements in the form of grids or built-up elements for forming a unit or module inside the apparatus for mass or heat transfer
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J23/00—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00
- B01J23/38—Catalysts comprising metals or metal oxides or hydroxides, not provided for in group B01J21/00 of noble metals
- B01J23/48—Silver or gold
- B01J23/52—Gold
-
- B01J35/23—
-
- B01J35/56—
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/0201—Impregnation
- B01J37/0207—Pretreatment of the support
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/0201—Impregnation
- B01J37/0211—Impregnation using a colloidal suspension
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/0215—Coating
- B01J37/0217—Pretreatment of the substrate before coating
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J37/00—Processes, in general, for preparing catalysts; Processes, in general, for activation of catalysts
- B01J37/02—Impregnation, coating or precipitation
- B01J37/0215—Coating
- B01J37/0225—Coating of metal substrates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y30/00—Nanotechnology for materials or surface science, e.g. nanocomposites
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y40/00—Manufacture or treatment of nanostructures
-
- C—CHEMISTRY; METALLURGY
- C01—INORGANIC CHEMISTRY
- C01B—NON-METALLIC ELEMENTS; COMPOUNDS THEREOF; METALLOIDS OR COMPOUNDS THEREOF NOT COVERED BY SUBCLASS C01C
- C01B32/00—Carbon; Compounds thereof
- C01B32/15—Nano-sized carbon materials
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B11/00—Single-crystal growth by normal freezing or freezing under temperature gradient, e.g. Bridgman-Stockbarger method
- C30B11/04—Single-crystal growth by normal freezing or freezing under temperature gradient, e.g. Bridgman-Stockbarger method adding crystallising materials or reactants forming it in situ to the melt
- C30B11/08—Single-crystal growth by normal freezing or freezing under temperature gradient, e.g. Bridgman-Stockbarger method adding crystallising materials or reactants forming it in situ to the melt every component of the crystal composition being added during the crystallisation
- C30B11/12—Vaporous components, e.g. vapour-liquid-solid-growth
-
- C—CHEMISTRY; METALLURGY
- C30—CRYSTAL GROWTH
- C30B—SINGLE-CRYSTAL GROWTH; UNIDIRECTIONAL SOLIDIFICATION OF EUTECTIC MATERIAL OR UNIDIRECTIONAL DEMIXING OF EUTECTOID MATERIAL; REFINING BY ZONE-MELTING OF MATERIAL; PRODUCTION OF A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; SINGLE CRYSTALS OR HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; AFTER-TREATMENT OF SINGLE CRYSTALS OR A HOMOGENEOUS POLYCRYSTALLINE MATERIAL WITH DEFINED STRUCTURE; APPARATUS THEREFOR
- C30B29/00—Single crystals or homogeneous polycrystalline material with defined structure characterised by the material or by their shape
- C30B29/60—Single crystals or homogeneous polycrystalline material with defined structure characterised by the material or by their shape characterised by shape
- C30B29/602—Nanotubes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J21/00—Catalysts comprising the elements, oxides, or hydroxides of magnesium, boron, aluminium, carbon, silicon, titanium, zirconium, or hafnium
- B01J21/06—Silicon, titanium, zirconium or hafnium; Oxides or hydroxides thereof
- B01J21/08—Silica
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J21/00—Catalysts comprising the elements, oxides, or hydroxides of magnesium, boron, aluminium, carbon, silicon, titanium, zirconium, or hafnium
- B01J21/18—Carbon
- B01J21/185—Carbon nanotubes
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00761—Details of the reactor
- B01J2219/00763—Baffles
- B01J2219/00765—Baffles attached to the reactor wall
- B01J2219/0077—Baffles attached to the reactor wall inclined
- B01J2219/00772—Baffles attached to the reactor wall inclined in a helix
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/32—Details relating to packing elements in the form of grids or built-up elements for forming a unit of module inside the apparatus for mass or heat transfer
- B01J2219/324—Composition or microstructure of the elements
- B01J2219/32466—Composition or microstructure of the elements comprising catalytically active material
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/32—Details relating to packing elements in the form of grids or built-up elements for forming a unit of module inside the apparatus for mass or heat transfer
- B01J2219/324—Composition or microstructure of the elements
- B01J2219/32491—Woven or knitted materials
-
- B01J35/31—
-
- B01J35/58—
-
- D—TEXTILES; PAPER
- D01—NATURAL OR MAN-MADE THREADS OR FIBRES; SPINNING
- D01F—CHEMICAL FEATURES IN THE MANUFACTURE OF ARTIFICIAL FILAMENTS, THREADS, FIBRES, BRISTLES OR RIBBONS; APPARATUS SPECIALLY ADAPTED FOR THE MANUFACTURE OF CARBON FILAMENTS
- D01F9/00—Artificial filaments or the like of other substances; Manufacture thereof; Apparatus specially adapted for the manufacture of carbon filaments
Definitions
- the present invention relates to nanowires, and more particularly, to nanowire manufacturing.
- the present invention also relates to the production of high density, high numbers of nanowires.
- Bulk nanowires have enormous potential in electrical, mechanical, and electro-mechanical applications.
- bulk nanowires can be used to make coatings for medical devices and as catalyst supports in fuel cell applications.
- a major impediment to commercializing devices using bulk nanowires is the ability to mass produce the nanowires used in such devices.
- Nanowires have been grown in chemical vapor deposition (CVD) furnaces primarily on glass and silicon wafers.
- CVD chemical vapor deposition
- Methods for increasing the quantity of nanowires grown per volume of furnace have focused on nanowires grown from powders, such as carbon black particles, using a fixed or fluidized bed and column approach. While powders provide a large surface area for nanowire growth, the particles may spill out of the column, and the precursor gases used may not flow uniformly through the column. In addition, non-uniform gas flow may cause silicon deposits to form on the particles, thereby causing the particles to stick together, leaving little space on the particles for nanowire growth.
- the present invention provides methods and apparatus for growing nanowires in a bulk or bulk-like process.
- the methods and apparatus of the present invention are scalable to the volume of the nanowire growth chamber that is available, and allow for a high density of nanowires to be efficiently produced within the given volume.
- the present invention provides methods for producing nanowires.
- a cartridge assembly having a plurality of support layers is provided, and the cartridge assembly is placed in a nanowire growth chamber.
- a spacing between adjacent support layers is a set distance, and each support layer has opposing first and second surfaces.
- a plurality of catalysts are disposed on at least the first surface of each layer.
- the methods further include providing a precursor gas in the nanowire growth chamber, whereby nanowires grow on the first surface of each layer.
- the precursor gas flows in a direction parallel to the surfaces and through the spacing between the support layers.
- the method may further include removing the cartridge assembly from the nanowire growth chamber and harvesting the nanowires.
- the present invention also provides methods for making a nanowire growth cartridge assembly.
- a sheet of material comprising spacers, each of a set height.
- the sheet of material is formed into a coiled sheet of material having a longitudinal axis and a plurality of support layers extending around the longitudinal axis.
- the plurality of support layers each have opposing first and second surfaces and are spaced apart by distances corresponding to the heights of the spacers.
- a plurality of catalysts are disposed on at least the first surface of each support layer.
- the sheet of material is embossed to generate the spacers.
- the nanowire growth cartridge assembly comprises a plurality of support layers, wherein each support layer has opposing first and second surfaces. A plurality of catalysts are disposed on at least the first surface of each support layer. A spacing between adjacent support layers is a set distance.
- a coiled sheet of material forms the support layers. The coiled sheet has a longitudinal axis, wherein the support layers extend around the longitudinal axis so as to be spaced apart by the set distance.
- the coiled sheet of material comprises integrated spacers embossed on the coiled sheet, having a height substantially equal to the set distance between the support layers. The spacers are configured to space apart the support layers by the set distance.
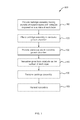
- FIG. 1 is a flow chart depicting a method for producing nanowires, in accordance with an embodiment of the present invention.
- FIG. 2 is a schematic illustration showing a cartridge assembly in a nanowire growth chamber, in accordance with an embodiment of the present invention.
- FIG. 3 is a schematic illustration of a cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 4 is a schematic illustration of a still further cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 5 is a schematic illustration showing an additional cartridge assembly in a nanowire growth chamber, in accordance with an embodiment of the present invention.
- FIG. 6A is an enlarged view of a portion of a mesh screen of a cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 6B is an enlarged view of the mesh screen of FIG. 6A , illustrating nanowires on its surfaces.
- FIG. 7 is a schematic illustration of the mesh screen of FIG. 6A , illustrating embodiments of directions of precursor gas flow.
- FIG. 8 is a schematic illustration showing a portion of a cartridge assembly in a nanowire growth chamber, in accordance with an embodiment of the present invention.
- FIG. 9 is a flow chart depicting a method for making a nanowire growth cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 10 is a schematic illustration showing a sheet of material with spacers, in accordance with an embodiment of the present invention.
- FIG. 11 is an axial view of the cartridge assembly of FIG. 2 prepared from the sheet of material of FIG. 10 .
- FIG. 12 is an axial view of a further cartridge assembly in accordance with an embodiment of the present invention.
- FIG. 13 is a schematic illustration of a support layer surface having catalysts disposed thereon in a pattern.
- FIG. 14 is a schematic illustration of an additional cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 15 is a schematic illustration of an additional cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 16 shows an exemplary process for producing nanowires in accordance with an embodiment of the present invention.
- FIG. 17 illustrates an exemplary cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 18 is a schematic illustration of an exemplary embossing apparatus used in making a cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 19 is a schematic illustration of an exemplary apparatus for treating surfaces of a cartridge assembly prior to disposing catalysts thereon, in accordance with an embodiment of the present invention.
- FIG. 20 is a schematic illustration of an exemplary apparatus for disposing catalysts on surfaces of a cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 21 is a schematic illustration showing a cartridge assembly in a nanowire growth chamber, in accordance with an embodiment of the present invention.
- FIG. 22 is a schematic illustration of an exemplary apparatus for harvesting nanowires from a cartridge assembly, in accordance with an embodiment of the present invention.
- FIG. 23 is a side view of an exemplary cartridge assembly in a nanowire growth chamber, in accordance with an embodiment of the present invention.
- FIG. 24 is an axial view of the cartridge assembly of FIG. 23 .
- FIGS. 25A-25B show scanning electron microscopy (SEM) micrographs of 60 nm gold nanoparticles disposed on a boehmite alumina substrate surface, at low magnification ( FIG. 25A ) and high magnification ( FIG. 25B ).
- FIGS. 26A-26C show SEM micrographs of 60 nm nanowires grown on an alumina substrate surface, at low magnification ( FIGS. 26A and 26B ) and high magnification ( FIG. 26C ).
- FIGS. 27A-27B show SEM micrographs of 20 nm gold nanoparticles disposed on a boehmite alumina substrate surface, at low magnification ( FIG. 27A ) and high magnification ( FIG. 27B ).
- FIGS. 28A-28D show SEM micrographs of 60 nm nanowires grown on an alumina substrate surface, at low magnification ( FIGS. 28A and 28B ) and high magnification ( FIGS. 28C and 28D ).
- nanowire generally refers to any elongated conductive or semiconductive material (or other material described herein) that includes at least one cross-sectional dimension that is less than about 1 ⁇ m.
- a nanowire produced according to the present invention will be less than about 500 nm, less than about 300 nm, less than about 200 nm, and less than about 100 nm in diameter.
- nanowires of the present invention have an aspect ratio (length:width) of greater than about 10, suitably greater than about 50, and more suitably greater than about 100.
- nanowires include semiconductor nanowires as described in Published International Patent Application Nos. WO 02/17362, WO 02/48701, and WO 01/03208, carbon nanotubes, and other elongated conductive or semiconductive structures of like dimensions.
- nanowire materials include CdS and Si
- other types of materials for nanowires can be used, including semiconductive nanowires, that are comprised of semiconductor material selected from, e.g., Si, Ge, Sn, Se, Te, B, C (including diamond), P, B—C, B—P(BP6), B—Si, Si—C, Si—Ge, Si—Sn and Ge—Sn, SiC, BN, BP, BAs, MN, AlP, AlAs, AlSb, GaN, GaP, GaAs, GaSb, InN, InP, InAs, InSb, ZnO, ZnS, ZnSe, ZnTe, CdS, CdSe, CdTe, HgS, HgSe, HgTe, BeS, BeSe, BeTe, MgS, MgSe, GeS, GeSe, GeTe, SnS, SnSe, SnTe, PbO, PbS,
- the semiconductor may comprise a dopant from a group consisting of: a p-type dopant from Group III of the periodic table; an n-type dopant from Group V of the periodic table; a p-type dopant selected from a group consisting of: B, Al and In; an n-type dopant selected from a group consisting of: P, As and Sb; a p-type dopant from Group II of the periodic table; a p-type dopant selected from a group consisting of: Mg, Zn, Cd and Hg; a p-type dopant from Group IV of the periodic table; a p-type dopant selected from a group consisting of: C and Si; or an n-type dopant selected from a group consisting of: Si, Ge, Sn, S, Se and Te.
- a dopant from a group consisting of: a p-type dopant from Group III of the periodic table an n-type dopant
- the nanowires can include carbon nanotubes, or nanotubes formed of conductive or semiconductive organic polymer materials, (e.g., pentacene, and transition metal oxides).
- conductive or semiconductive organic polymer materials e.g., pentacene, and transition metal oxides.
- Nanowire is referred to throughout the description herein for illustrative purposes, it is intended that the description herein also encompass the use of nanotubes (e.g., nanowire-like structures having a hollow tube formed axially therethrough). Nanotubes can be formed in combinations/thin films of nanotubes as is described herein for nanowires, alone or in combination with nanowires, as may be desired for a particular application.
- the nanowires grown on a substrate can be a “heterogeneous” so as to incorporate nanowires and/or nanotubes, and/or nanorods, and/or nanoribbons, and/or any combination thereof of different composition and/or structural characteristics.
- a “heterogeneous film” of nanowires can include nanowires/nanotubes with varying diameters and lengths, and nanotubes and/or nanotubes that are “heterostructures” having varying characteristics.
- the term “dispose” is used herein in connection with catalysts to indicate that the catalysts are generated, deposited, coated, applied, layered, sprayed or otherwise placed in contact with a surface or substrate.
- FIG. 1 shows a flow chart 100 of a method for producing nanowires in accordance with an embodiment of the present invention.
- a cartridge assembly is provided having a plurality of support layers, which serve as substrates for nanowire growth, with catalysts disposed on a surface of each layer.
- the cartridge is placed in a nanowire growth chamber.
- FIGS. 2-5 , 7 , 8 , and 11 - 13 which will be described in further detail below, show several non-limiting embodiments of cartridge assemblies for nanowire growth and examples of the placement of a cartridge assembly in a nanowire growth chamber.
- the method of flow chart 100 further includes providing one or more precursor gases in the growth chamber (step 130 ), whereby nanowires grow from the catalysts on the support layers (step 140 ).
- the method of flowchart 100 may further include the steps of removing the cartridge assembly from the nanowire growth chamber (step 150 ), and harvesting the nanowires (step 160 ).
- CVD chemical vapor deposition
- the catalysts are contacted with one or more precursor gas mixtures to initiate and promote nanowire growth from catalysts on the surface of each support layer (see steps 130 and 140 of flowchart 100 of FIG. 1 ).
- CVD comprises heating a precursor gas mixture to a temperature at which 1) the gas dissociates into its free component atoms, and 2) catalysts (e.g., metal film or colloids) melts to a liquid.
- catalysts e.g., metal film or colloids
- Such methods are also known as vapor-liquid-solid (VLS) synthesis methods.
- Catalysts that may be used in the practice of the present invention include metal catalysts, metal colloids and metal films, and can be any metal that can react with precursor gas mixtures to form a eutectic phase.
- a phase has a minimum melting point at which all components are in solution.
- precursor gas molecules e.g., silicon
- a saturation point on the eutectic phase diagram is reached such that semiconductor particles (e.g., Si) begin to precipitate out of the metal solution, thereby creating a growing nanowire.
- Continuous addition of precursor gas will continue to saturate the eutectic, thereby generating additional material for nanowire growth.
- the catalysts will be metal films and can comprise any of the transition metals from the Periodic Table, including, but not limited to, copper, silver, gold, nickel, palladium, platinum, cobalt, rhodium, iridium, iron, ruthenium, tin, osmium, manganese, chromium, molybdenum, tungsten, vanadium, niobium, tantalum, titanium, zirconium and gallium, including mixtures of one or more of these metals.
- the metal films are gold (Au) films.
- metallic colloids such as gold particles, can be used. In certain embodiments, the metallic colloids will be on the order of 10's of nanometers in diameter, for example, about 60 nanometer (nm) diameter gold colloids can be used. Other diameter colloids are envisioned.
- catalysts comprise metals, e.g., gold, and may be electroplated or evaporated onto the surface of the support layer or disposed in any of a number of other well known metal deposition techniques, e.g., sputtering, spraying, dip-coating etc.
- metal deposition techniques e.g., sputtering, spraying, dip-coating etc.
- catalysts used can be disposed on a support layer by heating a gold film layer coating the top surface of the substrate.
- the catalysts can be formed as metallic colloids using methods known in the art (see e.g., U.S. Pat. Nos. 7,105,428 and 7,067,867, both of which are incorporated by reference herein in their entireties).
- the colloids are typically disposed by first treating the surface of the substrate so that the colloids adhere to the surface. Such treatments include those known in the art, e.g., polylysine treatment, etc.
- the support layer with the treated surface is then immersed in a suspension of colloid.
- atomic layer deposition ALD is used for disposing alumina on a metal (such as stainless steel) support layer, and then the support layer is boiled to convert the alumina to ensure it is positively charged.
- the treated surface is then immersed in a suspension of colloid.
- the negatively charged metallic colloids are electrostaticly attracted to the positively charged alumina and disposed thereon.
- Metallic colloids can also be disposed using methods such as polydimethylsiloxane patterning, followed by contact with metallic colloids, as known in the art.
- nanowires can be grown directly on a surface of each support layer using a colloidal catalyst based vapor-liquid-solid (VLS) synthesis method.
- Colloidal catalysts are disposed upon the desired surface, or portion thereof, of a support layer (which in some cases may include both opposing surfaces of a support layer).
- the support layer including the colloidal catalyst is then subjected to the synthesis process which generates nanowires attached to the support layer surface.
- Other synthetic methods include the use of thin catalyst films, e.g., 50 nm, disposed over a surface of the support layer.
- the heat of the VLS process then melts the film to form small droplets of catalyst that form the nanowires. Typically, this latter method may be employed where nanowire diameter homogeneity is less critical to the ultimate application.
- the precursor gases can comprise a gas which includes at least one atomic species that promotes the growth of nanowires (e.g., Si) as well as an atomic species that aids in orienting the nanowires during their growth (e.g., Cl atoms).
- the first precursor gas may be selected from, but not limited to, Si 2 H 6 , SiH 4 , SiCl 4 and SiH 2 Cl 2 gas, preferably SiCl 4 or SiH 2 Cl 2 . Heating these Si precursor gases above the temperature at which the thermal energy is sufficient to break the bond energies between the gaseous molecules generates free Si atoms.
- Dissociation temperatures for SiH 4 and Si 2 H 6 , and SiCl 4 and SiH 2 Cl 2 gases are between about 300° C. to about 500° C. (for Si 2 H 6 and SiH 4 ), above about 800° C. (SiCl 4 ) and above about 600° C. (SiH 2 Cl 2 ) respectively.
- the metal catalyst following the initiation of nanowire growth and orientation with SiCl 4 or SiH 2 Cl 2 , it is suitable to introduce another precursor gas mixture (including, e.g., Si 2 H 6 or SiH 4 ) to contact the metal, which gas mixture includes a precursor gas which decomposes into Si atoms at lower temperatures than the first precursor gas (but at a high enough temperature to form a eutectic phase with the metal catalyst).
- another precursor gas mixture including, e.g., Si 2 H 6 or SiH 4
- the present invention also encompasses the use of multiple precursor gas mixtures added in different combinations and at different temperatures.
- the precursor gas mixtures used during any of the nanowire growth processes may further comprise one or more doping gases.
- doping gases examples include, but are not limited to, B 2 H 6 , POCl 3 and PH 3 . Further disclosure of VLS processes and use of various precursor gas mixtures and temperatures can be found in U.S. Pat. No. 7,105,428, which is incorporated herein by reference.
- FIG. 2 illustrates a cartridge assembly 220 in a nanowire growth chamber 230 in accordance with one embodiment of the present invention.
- Cartridge assembly 220 includes a coiled sheet of material having a plurality (n) of support layers 224 a, b, c . . . n , in which plurality of support layers 224 are spaced apart by a set distance 226 .
- “plurality” refers to two or more of an article (i.e., 2, 3, 4, 5, 10, 20, 30, 50, 100, 500, 1000 etc.).
- Each support layer 224 has opposing first and second surfaces (see surfaces 1012 and 1014 of FIGS. 10 and 11 ) on which a plurality of catalysts are disposed (see catalysts 442 illustrated in FIG.
- cartridge assembly 220 is cylindrical, having a diameter D, a length L, and a longitudinal axis 222 .
- FIG. 2 shows cartridge assembly 220 inserted into growth chamber 230 , such as a CVD furnace, so that longitudinal axis 222 of cartridge assembly 220 is parallel with the longitudinal axis of growth chamber 230 .
- nanowire growth chamber 230 has a cross-sectional shape that is substantially the same as the cross-sectional shape of cartridge assembly 220 .
- nanowire growth chamber 230 has a diameter d that is only slightly larger than diameter D of cartridge assembly 220 .
- One or more precursor gases are provided in nanowire growth chamber 230 , and flow in a direction f (as shown by the arrows illustrated in FIG. 2 ) that is parallel with longitudinal axis 222 and the surfaces of support layers 224 . It should be noted that flow direction f can also be in the opposite direction from that shown in FIG. 2 .
- the “set distance” is a predetermined distance between support layers that can vary about +/ ⁇ 20% from the predetermined distance to account for manufacturing error. Thus, the actual distance between the support layers may be in a range of about +/ ⁇ 20% of the predetermined distance.
- Support layers 224 are preferably spaced apart substantially evenly, to ensure uniform exposure of the catalysts to the precursor gas flowing between the spaced-apart layers. In the embodiment shown in FIG. 11 , support layers 224 are spaced evenly apart (i.e., set distance 226 is substantially uniform), ensuring an even gas flow due to the uniformity of the spacing in the coil (i.e., cartridge assembly 220 ).
- a substantially uniform set distance means that the each set distance between adjacent support layers has a predetermined distance that is within about +/ ⁇ 20% of each other set distance between other adjacent support layers of the cartridge assembly.
- Cartridge assembly 220 has a cross-sectional shape that is substantially circular (see FIG. 11 ).
- substantially circular refers to a curved shape in which every point on the curve is an equal distance from the center of the cross-section (i.e., uniform radii), or in which these distances vary by no more than a few % to about 20% across the curved shape.
- a substantially circular cross-section also includes oval cross-sections.
- cartridge assembly 220 can have a cross-sectional shape that is substantially rectangular or polygonal, and can be formed in a shape that corresponds with the cross-sectional shape of a given nanowire growth chamber.
- Cartridge assembly 220 can be any diameter D and length L, limited only by the dimensions of growth chamber 230 in which it is ultimately to be used.
- diameter D may range between 1-200 cm in one embodiment, 5-100 cm in another embodiment, 5-70 cm in another embodiment, and 7-60 cm in another embodiment.
- length L may range between 1-200 cm in one embodiment, 3-150 cm in another embodiment, 5-75 cm in another embodiment, and 7-60 cm in another embodiment.
- a cartridge assembly of diameter D of 7.5 cm and length L of 10 cm which is formed from coiling a sheet of material having a thickness of about 1.27 ⁇ m (0.0005 inches) and with the plurality of support layers being separated by a set distance of 30 ⁇ m, will have a total surface area for nanowire growth approaching 10 m 2 .
- Nanowires will grow from the surfaces of support layers 224 (see nanowires 440 illustrated in FIG. 11 ).
- cartridge assembly 220 can be removed from growth chamber 230 , and the nanowires harvested, such as by sonication or mechanical means, or any other means known to one of skill in the art.
- Cartridge assembly 220 can then be cleaned and reused if desired, or the material of cartridge assembly can be recycled.
- cartridge assembly 220 provides easy handling, while still providing a high surface area in comparison with the conventional fluidized bed approach for growing a high density of nanowires.
- precursor gas may be provided in a first direction for a first period of time (i.e., at the top end of from the bottom end of cartridge assembly 220 as shown in FIG.
- Other optimization techniques may include, for example, manipulating the heating profile along the longitudinal axis 222 of cartridge assembly 220 as well as preheating the precursor gas prior to providing it in the growth chamber 230 .
- a cartridge assembly 320 is formed from a sheet of material coiled in a cone shape, in which there is a smaller diameter at one end of the coiled sheet than a diameter at an opposite end of the coiled sheet.
- cartridge assembly 320 is provided with a plurality of support layers 324 which are spaced apart at a smaller diameter end 320 b of the coiled sheet by a spacing distance (set distance) 326 b that is less than a spacing distance 326 a between support layers 324 at an opposite, larger diameter end 320 a .
- the precursor gas can be provided to flow parallel with longitudinal axis 222 of cartridge assembly 320 , in the direction f as shown by the arrows illustrated in FIG. 3 (or can be in the opposite direction).
- the precursor gas flows from larger diameter end 320 a to smaller diameter end 320 b .
- larger spacing distance 326 a between layers at larger diameter end 320 a ensures there is less resistance to gas flow by the growing nanowires.
- spacing distance 326 between adjacent layers 324 at smaller diameter end 320 b correlates with a reduced surface area of each layer 324 at smaller diameter end 320 b . Consequently, a reduced amount of the precursor gas may be required at smaller diameter end 320 b , than at larger diameter end 320 a .
- spacing distance 326 between layers at that section can be configured to be substantially uniform (resembling the uniform spacing illustrated in the axial view of cartridge assembly 220 of FIG. 11 ). The substantially uniform spacing ensures uniform exposure of the catalyst and growing wires to the precursor gas at a given cross section of the cartridge assembly 320 .
- a growth chamber may have a rectangular cross-section, rather than circular, and in this instance the methods of the present invention may include providing a cartridge assembly that has a corresponding rectangular shape.
- cartridge assembly 220 can have a rectangular cross-section (see, e.g., FIG. 12 , illustrating a rectangular cartridge assembly 1220 comprised of a coiled sheet of material forming a plurality of support layers 1224 ).
- a rectangular cartridge assembly has a plurality of parallel plates, as illustrated in FIGS. 4 and 5 .
- a cartridge assembly 420 includes a plurality (n) of parallel plates 424 a - d , . . . n, in which each plate 424 has opposing first and second surfaces 412 , 414 (see FIG. 5 ).
- parallel plates 424 are spaced apart by a set distance 426 a , and a plurality of catalysts 442 may be disposed on one or both surfaces 412 , 414 .
- Cartridge assembly 420 is inserted into a rectangular growth chamber 530 and oriented so that the precursor gas can flow parallel to surfaces 412 , 414 , in a direction f illustrated by the arrows (or can be in the opposite direction).
- the precursor gas will pass through the spacing between the plurality of parallel plates 424 and nanowires will grow from surfaces 412 , 414 on which catalysts were disposed.
- the set distance 426 between the parallel plates 424 is preferably substantially uniform, to ensure an even gas flow between the support layers and uniform exposure of the catalysts and growing wires 440 .
- cartridge assembly 420 can be removed from growth camber 530 , and nanowires 440 harvested.
- FIGS. 6A , 6 B, 7 and 8 illustrate exemplary mesh screens 624 provided as a support layers. Each screen 624 has opposing first and second surfaces 612 , 814 with a plurality of apertures 608 . A plurality of nanowire catalysts can be disposed on either or both surfaces 814 and 612 , as well as in each aperture 608 . As illustrated in the enlarged views of FIGS. 6A and 6B , nanowires 640 may grow on surface 612 of screen 624 (provided that catalysts are disposed thereon).
- Nanowires can also grow into an interior surface 606 of aperture 608 . Further, nanowires can grow on opposing surface 814 (see FIG. 8 ) (provided that catalysts are provided thereon). Thus, the mesh screen offers an increased surface area for possible nanowire growth, including not only surfaces 612 and 814 , but also interior surface 606 of each aperture 608 .
- a precursor gas may be provided in a flow direction f, so as to be parallel with the surface of each mesh screen 624 .
- the precursor gas may be provided perpendicular to surfaces 612 and 814 , in a flow direction f, as illustrated by its respective arrow in FIG. 7 .
- the precursor gas flow is likely to be more turbulent than in the former example, as it will be disrupted by screen 624 and forced to flow through apertures 608 .
- FIG. 8 A plurality of spaced-apart parallel mesh screens comprise a cartridge assembly that is placed in a nanowire growth chamber 830 . Precursor gas flows in direction f, perpendicular to surfaces 612 and 814 , through apertures 608 .
- FIG. 9 provides a flowchart of a method 900 for making a nanowire growth cartridge assembly 220 .
- Method 900 includes steps 910 , 920 and 930 .
- step 910 a sheet of material is embossed to generate spacers.
- step 920 the sheet of material is formed into a coiled sheet of material having a plurality of support layers that are spaced apart by the spacers.
- step 930 catalysts are disposed on one or both opposing surfaces of each support layer. Reference is made to FIG.
- Sheet of material 1000 is suitably placed through a stamping machine (not shown) to emboss surface 1014 to create a plurality of spaced protrusions, or nubs, which form spacers 1050 , protruding from opposing surface 1012 .
- the surface may be crimped to have a corrugated pattern or to have intermittent folds which can serve as spacers 1050 .
- sheet of material 1000 has been manipulated after embossing to form a coil so as to have the plurality of support layers 224 spaced apart by spacers 1050 .
- the heights 1052 of each spacer 1050 is substantially the same as the set distance 226 that separates adjacent support layers 224 , and ensures that support layers 224 are separated by set distance 226 .
- a sheet of material is easily formed to include spacers so that the sheet can be coiled to form support layers 224 that are guaranteed to be spaced apart by set distance.
- Spacing between support layers may range between 10-1000 lam in one embodiment, 20-500 ⁇ m in another embodiment, 20-100 ⁇ m in another embodiment, and 30-50 ⁇ m in another embodiment.
- the spacing between adjacent support layers 224 may be about 50 ⁇ m, with corresponding spacers of about 50 ⁇ m, and in another example the spacing 224 may be about 30 ⁇ m, with corresponding spacers of about 30 ⁇ m.
- spacers are provided on only a few percent of the surface area of sheet material 1000 , for example from 2-10%, from 3-8%, from 2-5%, or from less than 1%.
- heights 1052 of each spacer 1050 may be substantially the same, thereby ensuring a uniform spacing between plurality of support layers 224 of cartridge assembly 220 .
- the heights 1052 vary.
- the heights may vary along sheet of material 1000 in a configuration such that the spacing varies radially between adjacent support layers.
- the outer layers of the coil may be closer together than the inner layers, or vice versa.
- the heights of spacers may range from about one to about five times the length of the nanowires that are desired to be grown on the surface of the sheet of material 1000 .
- spacers 1050 may be used as spacers 1050 to separate support layers by the set distance.
- spacers 1050 may be used as spacers 1050 to separate support layers by the set distance.
- spacers 1050 may or may not be removed from the sheet of material after it is coiled into cartridge assembly 220 .
- catalysts can be disposed onto surfaces 1012 and/or 1014 of sheet material 1000 prior to coiling the sheet.
- sheet of material 1000 is coiled prior to disposing the catalysts.
- several methods for disposing catalysts onto nanowire growth substrates may be used.
- aluminum foil may be used as sheet of material 1000 . The foil is boiled for approximately 10 minutes, so as to convert the surface to aluminum trihydrate, and then the coil is dried and placed in a solution of gold colloid, which sticks to the surface. The aluminum coil is then dried off and the coil is tightened prior to being placed into the growth chamber (e.g., growth chamber 230 ).
- This process may also be made continuous, as a roll-to-roll process, in which sheet material 1000 is (i) unwound from coil 1100 (see FIG. 10 ), (ii) sent through an embossing machine to generate spacers 1050 integrally on surface 1012 of sheet 100 , (iii) boiled, (iv) dried, then (v) gold colloid is disposed thereon, then (vi) dried and (vii) recoiled, to form cartridge assembly 220 .
- catalysts may be disposed using polylysine as an adhesive layer, electrophoresis, or a gold film may be disposed on one or both surfaces 1012 , 1014 , and heat then applied that causes the colloid to coalesce and form droplets of catalysts.
- nanowires may grow over the course of a few hours. For example, one run using an 8 inch coil cartridge may produce 100-200 grams, whereby five runs produces about one kilogram of nanowires. For fuel cell catalysts, for example, a gram of nanowires is often needed. Thus, it should be apparent that bulk nanowire production is achieved by the apparatus and methods of the present invention.
- the coiled sheet may be unrolled prior to removal of the nanowires.
- the nanowires may be harvested directly from coiled sheet, with the sheet remaining coiled.
- Sonication or mechanical means, or any other means known to one of skill in the art may be used to harvest the nanowires.
- the sheet of material with nanowires is immersed in a solution bath (e.g., a bath of isopropyl alcohol (IPA)) and subjected to ultrasonic waves from ultrasonic transducers, whereby the nanowires are detached from the sheet of material and are collected in the bath.
- IPA isopropyl alcohol
- a subsequent filtration step may then be employed to separate the nanowires from the solution (IPA).
- the nanowires and IPA mixture may be filtered under pressure through a micron filter (e.g., 0.4 micron filter), with the nanowires being collected by the filter.
- the collected filter mat of nanowires may then be dried.
- An exemplary sonication method of harvesting the nanowires from the unrolled coiled sheet is described later with reference to FIG. 21 .
- Sheet of material 1000 is suitably as thin as possible to maximize the surface area available for a nanowire growth.
- a sheet of material may have a thickness in the range of less than about 1 mm, and in other embodiments, the sheet of material has a thickness of about 0.5 to about 1 mm, about 5 to about 50 ⁇ m, or about 10-20 ⁇ m.
- Sheet of material may be any suitable material including glass, polymers, ceramics or metals.
- Sheet of material 1000 is not required to be of crystalline structure, but should withstand a high temperature in a CVD furnace, as well as the temperature necessary for disposition of the catalysts.
- sheet of material 1000 may also be a flexible mesh wire mesh material that may be rolled into a coil.
- a stainless steel sheet is used that is 0.00127 cm thick and 10 cm wide. Such a sheet can be provided with 30 ⁇ m protrusion to make a cylindrical cartridge assembly having a 7.5 cm diameter and 10 centimeters in length, with the available surface area for nanowire growth approaching 10 m 2 .
- cartridge assembly 220 has a 7.62 cm diameter D and an 20.32 cm length L.
- the present invention is scalable, such that increasing the size of the cartridge assembly should increase the total weight of nanowires that may be harvested therefrom.
- an 20.32 cm diameter cartridge assembly 220 being about 0.3 m to 0.6 m in length may produce between 0.5 to 1 kilograms of nanowires in bulk.
- size of cartridge assembly 220 may be optimized for ease of handling. For example, it may be decided that it is more desirable to have multiple smaller tubes in a large growth chamber 230 , rather than one large tube. Further, there may be production issues that arise as the cartridge increases in size, such as, for example, heat uniformity issues.
- catalysts may be disposed on one or both opposing surfaces of each support layer of the cartridge assembly.
- the density of disposed catalysts may be selected as desired, such as, for example, 1 to 100 particles/cm 2 . In other embodiments, the density can be, for example, 1 to 500 particles/cm 2 , or 100 to 500 particles/cm 2 . Further, the catalysts can be disposed uniformly on each surface or may be disposed in a pattern.
- FIG. 13 illustrates an example of a patterned disposition of catalysts 1390 on a surface 1312 of an exemplary support layer 1324 .
- Support layer 1324 may be one of the plurality of support layers 424 of cartridge assembly 420 with parallel plates (see FIG.
- a masking 1321 is suitably provided on surface 1312 of support layer 1324 in the region where nanowire growth is not desired prior to disposing the catalyst on the surface.
- Masking 1321 may be any film or other material layer to which catalysts 1390 will not adhere to, such that catalysts 1390 are disposed only the surface of 1324 in the regions where masking 1321 is absent.
- a cartridge assembly 1420 in accordance with the present invention can be comprised of a plurality of parallel tubes 1424 .
- Each tube 1424 has an inner surface 1412 and an outer surface 1414 on which catalysts may be disposed.
- This cartridge of tubes 1424 may be placed in a nanowire growth chamber, and the precursor gas may be provided in a flow direction that is perpendicular to the longitudinal axes of tubes 1424 , or in a flow direction that is parallel with the longitudinal axes of tubes 1424 .
- the precursor gas may be provided in a flow direction that is perpendicular to the longitudinal axes of tubes 1424 , or in a flow direction that is parallel with the longitudinal axes of tubes 1424 .
- a cartridge assembly 1520 can be comprised of a plurality of concentric tubes 1524 , each surface of each tube 1524 serving as a support layer for nanowire growth.
- the cartridge assemblies of the present invention as nanowire growth supports, an efficient use of the precursor gas can be achieved and nanowire growth can be maximized for the available surface area and growth chamber volume.
- gas flow, gas concentration temperature, and gas flow directions may be optimized to ensure uniform exposure of the catalysts and growing nanowires, and efficient use of the precursor gas.
- flowchart 1600 provides exemplary production methods for nanowire manufacturing.
- the methods of flowchart 1600 suitably comprise step 1602 , in which an aluminum foil is embossed.
- step 1604 of flowchart 1600 the foil is then cleaned, and in step 1606 a substrate surface is prepared (e.g., a metal, polymer, carbon or other substrate).
- Gold colloid is disposed on the substrate in step 1608 , followed by drying in step 1610 .
- Nanowire growth is then performed in step 1612 using a VLS-process (other processes as described herein can also be used).
- the nanowires are then harvested (for example, by sonication) in step 1614 , filtered in step 1616 and dried in step 1618 .
- the nanowires can then be ball milled in step 1620 to break the nanowires into short segments, which may be used, for example, as additives in a battery slurry as described in U.S. Provisional Patent Application No. 61/221,392, filed Jun. 29, 2009, entitled, “Nanostructured Materials for Battery Applications,” the disclosure of which is incorporated by reference herein.
- the embossed aluminum foil having an embossed profile 1713 on its surface can be formed into a coil that serves as a cartridge assembly 1720 to facilitate preparation of a large number of nanowires, in accordance with the present invention.
- FIG. 18 shows an embossing apparatus 1880 which may be used for step 1602 to emboss the aluminum foil. Specifically, a coil of aluminum foil 1100 is unwound to form an uncoiled sheet of foil 1000 that is suitably placed through embossing apparatus 1880 to provide embossed profile 1713 on the foil's surface.
- Embossing apparatus 1880 includes a left-handed helical die roll 1881 , a right-handed helical die roll 1882 , and a nip roll 1884 .
- Foil 1000 is sandwiched between the rolls, with the die rolls 1881 , 1882 disposed against a first surface of foil 1000 and nip roll 1884 disposed against the opposing second surface of foil 1000 .
- a suitable pressure is applied by rolls 1881 , 1882 , and 1884 against the surfaces of foil 1000 such that foil 1000 is embossed to have embossed profile 1713 .
- Foil 1000 is rewound after embossing to form cartridge assembly 1720 .
- Embossed profile 1713 serves as spacers that maintain a substantially uniform space between layers of the coiled foil that form cartridge assembly 1720 .
- Cleaning of the foil in step 1604 may be achieved by immersing the foil in a standard chemical immersion apparatus.
- the foil may immersed in a bath of IPA.
- the surfaces of cartridge assembly 1720 may be prepared for disposing gold colloid thereon in step 1608 .
- Surface preparation of cartridge assembly 1720 may be achieved by subjecting cartridge assembly 1720 to a hot water treatment process using the apparatus illustrated in FIG. 19 . Hot water may be used to convert the cartridge's surfaces from metallic aluminum to hydroxylated or hydrated alumina, to which the gold colloid is attracted.
- FIG. 19 includes a reservoir 1960 provided with a heater 1964 and a vacuum 1966 , and a surface preparation tank 1970 in fluid communication with reservoir 1960 via an inlet valve 1962 and a return valve 1968 .
- Surface preparation tank 1970 is provided with a cover 1972 , a vacuum valve 1976 , and a stirrer 1977 for circulating hot water in the tank.
- cartridge assembly 1720 is placed in surface preparation tank 1970 and cover 1972 is closed.
- Vacuum 1966 is then operated to eliminated air bubbles in the water contained in reservoir 1960
- vacuum valve 1976 is operated to eliminate air bubbles trapped between foil layers in cartridge assembly 1720 .
- Both inlet valve 1962 and outlet valve 1968 are then opened, and the water, heated by heater 1964 , is circulated through surface preparation tank 1970 , entering surface preparation tank 1970 via inlet valve 1962 , and exiting via return valve 1968 .
- Disposing gold colloid (step 1608 ) on cartridge assembly 1720 may be achieved using the apparatus illustrated in FIG. 20 .
- This apparatus includes a reservoir 2060 provided with a vacuum 2066 , and a disposition tank 2070 in fluid communication with reservoir 2060 via an inlet valve 2062 and a return valve 2068 .
- Disposition tank 2070 is provided with a cover 2072 , a vacuum valve 2076 , and a stirrer 2077 for circulating gold colloid in the tank.
- cartridge assembly 1720 which has been surface-treated in step 1606 is disposed in tank 2070 and cover 2072 is closed.
- Vacuum 2066 is then operated to eliminated air bubbles in the gold colloid contained in reservoir 2060 , and vacuum valve 2076 is operated to eliminate any air bubbles trapped between foil layers in cartridge assembly 1720 .
- Both inlet valve 2062 and outlet valve 2068 are then opened, and the gold colloid is circulated through disposition tank 2070 , entering the tank via inlet valve 2062 , and exiting via return valve 2068 .
- cartridge assembly 1720 is dried (step 1610 ). Drying may be achieved by loading the cartridge assembly in a nanowire growth chamber, and flushing the chamber with high velocity hot air. Hot air may be used in combination with other heaters (such as a heating coil which heats the cartridge assembly in the chamber).
- a precursor gas is applied to the chamber to grow nanowires on the surfaces of each layer of cartridge assembly 1720 (step 1612 ).
- the chamber may be purged of the precursor gas and cartridge assembly 1720 cooled.
- FIG. 21 illustrates a schematic (shown in cross-section) of cartridge assembly 1720 loaded in an exemplary nanowire growth chamber 2130 for growing nanowires (step 1612 ).
- Chamber 2130 may also be used for the pre-growth process of drying and the post-growth process of cooling.
- Chamber 2130 includes an outer enclosure 2134 within which is disposed insulation 2136 , a gas heater coil 2184 , a blanket heater coil 2186 , and a process heater coil 2188 (each of which may be cooling coils if the chamber is intended for use in post-growth process cooling).
- Cartridge assembly 1720 is loaded at loading platform 2139 at the bottom of chamber 2130 , and a housing 2138 covers a top of cartridge assembly 1720 in chamber 2130 .
- a holding bolt 2199 holds cartridge assembly 1720 sandwiched between loading platform 2139 and top housing 2138 .
- a piston seal 2198 separates the heater coils from cartridge assembly 1720 , and a seal gasket 2196 provides a seal were loading platform 2139 connects to enclosure 2134 .
- Loading platform 2139 , top housing 2138 , and piston seal 2198 define an inner chamber in which cartridge assembly 1720 is disposed and through which gas flows.
- Blanket heater coil 2186 surrounds a top of cartridge assembly 1720 and process heater coil 2188 surrounds sides of cartridge assembly 1720 .
- the gas flow direction f through the chamber 2130 is illustrated by the arrows in FIG. 21 . As shown, the gas flow direction is parallel with a longitudinal axis of cartridge assembly 1720 .
- the gas used is a suitable precursor gas, whereby nanowires grow from gold colloid catalysts disposed on the layers of cartridge assembly 1720 . If chamber 2130 is used for the pre-growth process of drying (step 1610 ) or the post-growth process of cooling, then the gas used may be air, for example.
- FIG. 22 illustrates a schematic of an exemplary apparatus for harvesting nanowires from cartridge assembly 1720 (step 1614 ) using a sonication process.
- the harvesting apparatus includes a harvesting tank 2256 filled with a solvent such as IPA.
- Tank 2256 is placed in an outer tank 2258 filled with distilled H 2 O (DI-H 2 O).
- DI-H 2 O distilled H 2 O
- At the bottom of outer tank 2258 are one or more ultrasonic transducers 2259 .
- the tank within another tank is used for improving the safety of the sonication process, but is not necessary.
- cartridge assembly 1720 is unwound such that sheet of material 1000 with nanowires thereon (not shown) is passed through harvesting tank 2256 via multiple rollers 2291 , 2292 , 2293 , and 2294 .
- Rollers 2292 and 2293 reside within harvesting tank 2256 and serve to keep material 1000 immersed in the IPA while being subjected to the ultrasonic waves from transducers 2259 .
- Flat spray manifolds 2295 are provided to spray the opposing surfaces of material 1000 as it exits harvesting tank 2256 , so as to remove nanowires still connected to the of surface material 1000 after sonication.
- material 1000 is rewound into a coil 2220 which can be cleaned and reused as a cartridge assembly, or can be recycled.
- Nanowire growth trials were conducted using a process in accordance with the present invention.
- the process included 12 process stages, which include Aluminum foil substrate mechanical pre-patterning (stage 1); Pre-cleaning in IPA (stage 2); Hot distilled H 2 O (DI-H 2 O) hydroxylation/oxidation treatment (stage 3); Triple DI-H 2 O rinsing (stage 4); Gold nanoparticle disposition (stage 5); Triple DI-H 2 O rinsing (stage 6); IPA rinsing (stage 7); Oven drying (stage 8); CVD nanowire growth (stage 9); Wire harvesting (stage 10); Filtration (stage 11); and Drying (stage 12). Further details on the process stages is provided below. A summary of the trial data for Trial runs 202-051 and 202-057 is presented in Table 1.
- This stage involved producing sheets of aluminum (Al) with integral spacers which creates the necessary space between adjacent layers of sheet when coiled to ensure both liquid disposition of Au nanoparticles (from an Au colloid solution) and subsequent growth of Si NWs (from silane gas decomposition in a CVD furnace).
- Al aluminum
- one Al sheet was 8 inches (20.32 cm) wide, 2.0 mil thick, 1100 alloy hard-tempered “H19” (1100-H19 from AllFoils, Inc.) and was deformed while passing through stainless steel rollers to produce a corrugation pattern with amplitude of about 0.9 mm.
- a second Al sheet that was 6 inches (15.24 cm) wide was embossed with dots. The second Al sheet was placed on the first AL sheet, and the sheets were rolled together to form a single coil.
- stage 2 the coil is pre-cleaned in IPA, by placing the coil vertically in a bath of IPA and subjecting the bath to 170 KHz sonication.
- stage 3 the coiled is subjected to a hot hydroxylation/oxidation treatment.
- the coil is placed in a bath of distilled H 2 O (DI-H 2 O) at 60° C. whereby the Al substrate surfaces are chemically converted from metallic aluminum to hydroxylated or hydrated alumina to ensure they are positively charged.
- DI-H 2 O distilled H 2 O
- gold catalyst nanoparticles are disposed on the surfaces of the coil by soaking the coil in an aqueous suspensions of 0.01% Au chloride in DI-H 2 O.
- the negatively charged Au catalyst particles (HAuCl 4 ) in the colloid are electrostaticly attracted to the positively charged alumina coil surfaces and substantially uniformly deposit on the surfaces.
- the colloidal solutions may range between 10%-70% Au Colloid (0.01% Au Chloride) in DI-H 2 O.
- 60 nm size gold particles were disposed
- Trial run 202-057 20 nm size gold particles were disposed on the surfaces.
- FIGS. 25A and 25B show SEM micrographs of 60 nm Au nanoparticles on the alumina coil surface from Trial run 202-051.
- FIGS. 27A and 27B show SEM micrographs of 20 nm Au nanoparticles on the alumina coil surface from Trial run 202-057.
- a colloidal aqueous suspension (0.01% Au Chloride) available commercially from BB International (British Biocell) was diluted to a solution of 30% Au Colloid (0.01% Au Chloride) in DI-H 2 O.
- stage 6 the coil was tripled rinsed in DI-H 2 O, and in stage 7, the coil was rinsed in IPA. In stage 8, the coil was oven dried at 110° C.
- FIG. 23 illustrates an Au-nanoparticle treated corrugated coil 2320 being bottom-loaded into a furnace tube 2330 , in accordance with the present Example.
- Holding bolt 2199 extends along the center axis of the coil 2320 .
- a foil piece may be placed above holding bolt 2199 and used as a spacer to center the coil at the hot zone center (not shown) of the elongated furnace tube 2330 .
- FIG. 24 illustrates an axial view of coil 2320 with holding bolt 2199 .
- silane gas SiH 4
- the gold nanoparticles act as catalytic sites or seeds for the thermal, gas-phase CVD growth of crystalline silicon wires of the same diameter.
- Catalyst diameter determines nanowire diameter, and wire length is controlled by exposure time to the silane gas.
- nanowire growth rate is about 0.7 ⁇ m/min.
- FIGS. 26A-26C show SEM micrographs of 60 nm NWs grown on a coil surface, from Trial run 202-051.
- FIGS. 28A-28D show SEM micrographs of 20 nm NW grown on a coil surface from Trial run 202-057.
- the nanowires are ultrasonically harvested (40 kHz sonication) by unwinding the coil and passing the unwound sheet of material through a bath of Di-H2O-10% IPA solution.
- the unwound sheet is placed close to a bottom surface of the bath in a horizontal orientation.
- the harvested 60 nm Si NWs in Di-H2O-10% IPA solution from had a yellow color
- the solution of 20 nm Si NWs harvested in Di-H2O-10% IPA solution had a brown color.
- stage 11 the NWs were filtered from the Di-H2O-10% IPA solution using a laboratory vacuum filter flask having a paper filter.
- stage 11 the NWs captured by the filter paper were dried and then separated from the filter paper.
Abstract
Description
| TABLE 1 | |||
| Run# 202- | |||
| -051 | -057 | |||
| 1100-H19 Al foils length (6″ & 8″): | ~11.1 ft | ~9.6 ft | ||
| Au Colloid size (nm) | 60 | 20 | ||
| Au Colloid Deposit Time (hrs in | 5.0 | 4.5 | ||
| diluted 0.01% Au Chloride)** | ||||
| Au NP density (per μm2) | 10.6 | 21.2 | ||
| Tube OD (in) | 3 | 3 | ||
| Surface area (m2): | 2.42 | 2.07 | ||
| NW Growth conditions: | ||||
| Total Pressure (torr): | 30 | 45 | ||
| Silane Partial Pressure (torr): | 1.7 | 4.8 | ||
| Silane Flow (sccm) | 50.2 | 75.2 | ||
| Hydrogen Flow (sccm) | 301 | 234 | ||
| Helium Flow (sccm) | 551 | 401 | ||
| TC Setpoint (° C.) | 455 | 450 | ||
| Total Gas Flow (sccm) | 902 | 711 | ||
| Calc. Gas Residence Time (sec)*** | 0.77 | 1.48 | ||
| Growth Time (min) | 60 | 100 | ||
| NW + Si Deposit wt gain (g): | 2.52 | 1.98 | ||
| (g/m2) | 1.04 | 0.96 | ||
| **Dilutions are 60% for 60 nm and 30% for 20 nm. | ||||
| ***neglecting cross-sectional area occupied by the foils | ||||
Claims (13)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/824,485 US8623288B1 (en) | 2009-06-29 | 2010-06-28 | Apparatus and methods for high density nanowire growth |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US22150109P | 2009-06-29 | 2009-06-29 | |
| US12/824,485 US8623288B1 (en) | 2009-06-29 | 2010-06-28 | Apparatus and methods for high density nanowire growth |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US8623288B1 true US8623288B1 (en) | 2014-01-07 |
Family
ID=49840828
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/824,485 Active 2032-11-07 US8623288B1 (en) | 2009-06-29 | 2010-06-28 | Apparatus and methods for high density nanowire growth |
Country Status (1)
| Country | Link |
|---|---|
| US (1) | US8623288B1 (en) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090255222A1 (en) * | 2007-07-10 | 2009-10-15 | Raul Cortez | Methods and apparatus for the in situ collection of nucleated particles |
| US20130092525A1 (en) * | 2007-07-10 | 2013-04-18 | Innovalight, Inc. | Concentric flow-through plasma reactor and methods therefor |
| US9812699B2 (en) | 2011-10-05 | 2017-11-07 | Oned Material Llc | Silicon nanostructure active materials for lithium ion batteries and processes, compositions, components and devices related thereto |
| US10243207B2 (en) | 2011-07-26 | 2019-03-26 | Oned Material Llc | Nanostructured battery active materials and methods of producing same |
| US10862114B2 (en) | 2016-07-15 | 2020-12-08 | Oned Material Llc | Manufacturing apparatus and method for making silicon nanowires on carbon based powders for use in batteries |
Citations (118)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5196396A (en) | 1991-07-16 | 1993-03-23 | The President And Fellows Of Harvard College | Method of making a superconducting fullerene composition by reacting a fullerene with an alloy containing alkali metal |
| US5332910A (en) | 1991-03-22 | 1994-07-26 | Hitachi, Ltd. | Semiconductor optical device with nanowhiskers |
| US5505928A (en) | 1991-11-22 | 1996-04-09 | The Regents Of University Of California | Preparation of III-V semiconductor nanocrystals |
| US5512131A (en) | 1993-10-04 | 1996-04-30 | President And Fellows Of Harvard College | Formation of microstamped patterns on surfaces and derivative articles |
| US5674592A (en) | 1995-05-04 | 1997-10-07 | Minnesota Mining And Manufacturing Company | Functionalized nanostructured films |
| US5690807A (en) | 1995-08-03 | 1997-11-25 | Massachusetts Institute Of Technology | Method for producing semiconductor particles |
| US5840435A (en) | 1993-07-15 | 1998-11-24 | President And Fellows Of Harvard College | Covalent carbon nitride material comprising C2 N and formation method |
| US5858862A (en) | 1996-09-25 | 1999-01-12 | Sony Corporation | Process for producing quantum fine wire |
| US5897945A (en) | 1996-02-26 | 1999-04-27 | President And Fellows Of Harvard College | Metal oxide nanorods |
| US5962863A (en) | 1993-09-09 | 1999-10-05 | The United States Of America As Represented By The Secretary Of The Navy | Laterally disposed nanostructures of silicon on an insulating substrate |
| US5976957A (en) | 1996-10-28 | 1999-11-02 | Sony Corporation | Method of making silicon quantum wires on a substrate |
| US5997832A (en) | 1997-03-07 | 1999-12-07 | President And Fellows Of Harvard College | Preparation of carbide nanorods |
| US6036774A (en) | 1996-02-26 | 2000-03-14 | President And Fellows Of Harvard College | Method of producing metal oxide nanorods |
| US6048616A (en) | 1993-04-21 | 2000-04-11 | Philips Electronics N.A. Corp. | Encapsulated quantum sized doped semiconductor particles and method of manufacturing same |
| US6068800A (en) | 1995-09-07 | 2000-05-30 | The Penn State Research Foundation | Production of nano particles and tubes by laser liquid interaction |
| US6132874A (en) | 1995-08-22 | 2000-10-17 | Rhodia Chimie | Functionalized inorganic oxide hydroxylated carrier and method for preparing same |
| US6136156A (en) | 1996-03-01 | 2000-10-24 | Virginia Commonwealth University | Nanoparticles of silicon oxide alloys |
| US6146227A (en) | 1998-09-28 | 2000-11-14 | Xidex Corporation | Method for manufacturing carbon nanotubes as functional elements of MEMS devices |
| US6190634B1 (en) | 1995-06-07 | 2001-02-20 | President And Fellows Of Harvard College | Carbide nanomaterials |
| US6207229B1 (en) | 1997-11-13 | 2001-03-27 | Massachusetts Institute Of Technology | Highly luminescent color-selective materials and method of making thereof |
| US6225198B1 (en) | 2000-02-04 | 2001-05-01 | The Regents Of The University Of California | Process for forming shaped group II-VI semiconductor nanocrystals, and product formed using process |
| US6235675B1 (en) | 1998-09-22 | 2001-05-22 | Idaho Research Foundation, Inc. | Methods of forming materials containing carbon and boron, methods of forming catalysts, filaments comprising boron and carbon, and catalysts |
| US6256767B1 (en) | 1999-03-29 | 2001-07-03 | Hewlett-Packard Company | Demultiplexer for a molecular wire crossbar network (MWCN DEMUX) |
| US6261469B1 (en) | 1998-10-13 | 2001-07-17 | Honeywell International Inc. | Three dimensionally periodic structural assemblies on nanometer and longer scales |
| US6274007B1 (en) | 1999-11-25 | 2001-08-14 | Sceptre Electronics Limited | Methods of formation of a silicon nanostructure, a silicon quantum wire array and devices based thereon |
| US6294450B1 (en) | 2000-03-01 | 2001-09-25 | Hewlett-Packard Company | Nanoscale patterning for the formation of extensive wires |
| JP2001288626A (en) | 2000-04-03 | 2001-10-19 | Nissan Motor Co Ltd | Method for producing fixed body of carbon nanofiber |
| US6306736B1 (en) | 2000-02-04 | 2001-10-23 | The Regents Of The University Of California | Process for forming shaped group III-V semiconductor nanocrystals, and product formed using process |
| US6306734B1 (en) | 1996-04-01 | 2001-10-23 | Evgeny Invievich Givargizov | Method and apparatus for growing oriented whisker arrays |
| US20010033796A1 (en) | 1999-06-28 | 2001-10-25 | Unger Marc A. | Microfabricated elastomeric valve and pump sysems |
| US20010051367A1 (en) | 1999-04-14 | 2001-12-13 | Ching-Hwa Kiang | Molecular nanowires from single walled carbon nanotubes |
| US20020004136A1 (en) | 1999-06-14 | 2002-01-10 | Yufei Gao | Carbon nanotubes on a substrate |
| US6339281B2 (en) | 2000-01-07 | 2002-01-15 | Samsung Sdi Co., Ltd. | Method for fabricating triode-structure carbon nanotube field emitter array |
| US20020014667A1 (en) | 2000-07-18 | 2002-02-07 | Shin Jin Koog | Method of horizontally growing carbon nanotubes and field effect transistor using the carbon nanotubes grown by the method |
| US6380103B2 (en) | 1996-01-03 | 2002-04-30 | Micron Technology, Inc. | Rapid thermal etch and rapid thermal oxidation |
| US20020072577A1 (en) | 2000-10-04 | 2002-06-13 | The Dow Chemical Company | Supported catalyst compositions |
| US6413489B1 (en) | 1997-04-15 | 2002-07-02 | Massachusetts Institute Of Technology | Synthesis of nanometer-sized particles by reverse micelle mediated techniques |
| US20020127495A1 (en) | 2001-03-12 | 2002-09-12 | Axel Scherer | Method of fabricating nanometer-scale flowchannels and trenches with self-aligned electrodes and the structures formed by the same |
| US20020130311A1 (en) | 2000-08-22 | 2002-09-19 | Lieber Charles M. | Doped elongated semiconductors, growing such semiconductors, devices including such semiconductors and fabricating such devices |
| US20020130353A1 (en) | 1999-07-02 | 2002-09-19 | Lieber Charles M. | Nanoscopic wire-based devices, arrays, and methods of their manufacture |
| US6465132B1 (en) | 1999-07-22 | 2002-10-15 | Agere Systems Guardian Corp. | Article comprising small diameter nanowires and method for making the same |
| US6471761B2 (en) | 2000-04-21 | 2002-10-29 | University Of New Mexico | Prototyping of patterned functional nanostructures |
| US20020158342A1 (en) | 2001-03-14 | 2002-10-31 | Mark Tuominen | Nanofabrication |
| US20020172820A1 (en) | 2001-03-30 | 2002-11-21 | The Regents Of The University Of California | Methods of fabricating nanostructures and nanowires and devices fabricated therefrom |
| US20020179434A1 (en) | 1998-08-14 | 2002-12-05 | The Board Of Trustees Of The Leland Stanford Junior University | Carbon nanotube devices |
| US6495258B1 (en) | 2000-09-20 | 2002-12-17 | Auburn University | Structures with high number density of carbon nanotubes and 3-dimensional distribution |
| US6515325B1 (en) | 2002-03-06 | 2003-02-04 | Micron Technology, Inc. | Nanotube semiconductor devices and methods for making the same |
| US20030037675A1 (en) | 2000-09-05 | 2003-02-27 | Gillingham Gary R. | Air filtration arrangements having fluted media constructions and methods |
| US20030044777A1 (en) | 1993-10-28 | 2003-03-06 | Kenneth L. Beattie | Flowthrough devices for multiple discrete binding reactions |
| US20030071246A1 (en) | 2001-01-23 | 2003-04-17 | Grigorov Leonid N. | Quantum devices based on crystallized electron pairs and methods for their manufacture and use |
| US20030089899A1 (en) | 2000-08-22 | 2003-05-15 | Lieber Charles M. | Nanoscale wires and related devices |
| US6586785B2 (en) | 2000-06-29 | 2003-07-01 | California Institute Of Technology | Aerosol silicon nanoparticles for use in semiconductor device fabrication |
| US20030121887A1 (en) | 2001-03-22 | 2003-07-03 | Garvey James F. | Multi-component substances and processes for preparation thereof |
| US20030186522A1 (en) | 2002-04-02 | 2003-10-02 | Nanosys, Inc. | Methods of positioning and/or orienting nanostructures |
| US20030185741A1 (en) | 2001-04-06 | 2003-10-02 | Krzysztof Matyjaszewski | Process for the preparation of nanostructured materials |
| US20030184357A1 (en) | 2002-02-19 | 2003-10-02 | Commissariat A L'energie Atomique | Cathode structure with emissive layer formed on a resistive layer |
| US20030189202A1 (en) | 2002-04-05 | 2003-10-09 | Jun Li | Nanowire devices and methods of fabrication |
| US20030195611A1 (en) | 2002-04-11 | 2003-10-16 | Greenhalgh Skott E. | Covering and method using electrospinning of very small fibers |
| US20030200521A1 (en) | 2002-01-18 | 2003-10-23 | California Institute Of Technology | Array-based architecture for molecular electronics |
| US6656573B2 (en) | 2001-06-26 | 2003-12-02 | Hewlett-Packard Development Company, L.P. | Method to grow self-assembled epitaxial nanowires |
| US20030222019A1 (en) | 2002-05-28 | 2003-12-04 | Battelle Memorial Institute | Electrostatic deposition of particles generated from rapid expansion of supercritical fluid solutions |
| US20030232721A1 (en) | 2002-01-31 | 2003-12-18 | Hydrocarbon Technologies Inc. | Supported noble metal nanometer catalyst particles containing controlled (111) crystal face exposure |
| US20040005258A1 (en) | 2001-12-12 | 2004-01-08 | Fonash Stephen J. | Chemical reactor templates: sacrificial layer fabrication and template use |
| US20040026684A1 (en) | 2002-04-02 | 2004-02-12 | Nanosys, Inc. | Nanowire heterostructures for encoding information |
| US20040061422A1 (en) | 2002-09-26 | 2004-04-01 | International Business Machines Corporation | System and method for molecular optical emission |
| US20040063839A1 (en) | 2002-09-26 | 2004-04-01 | Canon Kabushiki Kaisha | Method of producing electron emitting device using carbon fiber, electron source and image forming apparatus, and ink for producing carbon fiber |
| US20040095658A1 (en) | 2002-09-05 | 2004-05-20 | Nanosys, Inc. | Nanocomposites |
| US20040112964A1 (en) | 2002-09-30 | 2004-06-17 | Nanosys, Inc. | Applications of nano-enabled large area macroelectronic substrates incorporating nanowires and nanowire composites |
| US20040121681A1 (en) | 2002-12-23 | 2004-06-24 | Kimberly-Clark Worldwide, Inc. | Absorbent articles containing an activated carbon substrate |
| US20040118448A1 (en) | 2002-09-05 | 2004-06-24 | Nanosys, Inc. | Nanostructure and nanocomposite based compositions and photovoltaic devices |
| US20040135951A1 (en) | 2002-09-30 | 2004-07-15 | Dave Stumbo | Integrated displays using nanowire transistors |
| US20040146560A1 (en) | 2002-09-05 | 2004-07-29 | Nanosys, Inc. | Oriented nanostructures and methods of preparing |
| US20040147098A1 (en) | 2002-11-22 | 2004-07-29 | Frederic Mazen | Method for forming, by CVD, nanostructures of semi-conductor material of homogeneous and controlled size on dielectric material |
| US6773616B1 (en) | 2001-11-13 | 2004-08-10 | Hewlett-Packard Development Company, L.P. | Formation of nanoscale wires |
| US6790425B1 (en) | 1999-10-27 | 2004-09-14 | Wiliam Marsh Rice University | Macroscopic ordered assembly of carbon nanotubes |
| US20040206448A1 (en) | 2003-04-17 | 2004-10-21 | Nanosys, Inc. | Structures, systems and methods for joining articles and materials and uses therefor |
| US6808605B2 (en) | 2001-10-15 | 2004-10-26 | Korea Institute Of Science And Technology | Fabrication method of metallic nanowires |
| US20040213307A1 (en) | 2002-07-19 | 2004-10-28 | President And Fellows Of Harvard College | Nanoscale coherent optical components |
| US6831017B1 (en) | 2002-04-05 | 2004-12-14 | Integrated Nanosystems, Inc. | Catalyst patterning for nanowire devices |
| US20050011431A1 (en) | 2003-04-04 | 2005-01-20 | Btg International Limited | Precisely positioned nanowhiskers and nanowhisker arrays and method for preparing them |
| US6846565B2 (en) | 2001-07-02 | 2005-01-25 | Board Of Regents, The University Of Texas System | Light-emitting nanoparticles and method of making same |
| WO2004032193A3 (en) | 2002-09-30 | 2005-01-27 | Nanosys Inc | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US20050026526A1 (en) | 2003-07-30 | 2005-02-03 | Verdegan Barry M. | High performance filter media with internal nanofiber structure and manufacturing methodology |
| US20050029678A1 (en) | 2003-07-08 | 2005-02-10 | University Of Texas System, Board Of Regents | Growth of single crystal nanowires |
| US20050038498A1 (en) | 2003-04-17 | 2005-02-17 | Nanosys, Inc. | Medical device applications of nanostructured surfaces |
| US20050062033A1 (en) | 2003-08-08 | 2005-03-24 | Canon Kabushiki Kaisha | Structure and method for production of the same |
| US20050066883A1 (en) | 2003-09-25 | 2005-03-31 | Nanosys, Inc. | Methods, devices and compositions for depositing and orienting nanostructures |
| US20050079659A1 (en) | 2002-09-30 | 2005-04-14 | Nanosys, Inc. | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US20050089467A1 (en) | 2003-10-22 | 2005-04-28 | International Business Machines Corporation | Control of carbon nanotube diameter using CVD or PECVD growth |
| US6888208B2 (en) | 2003-07-30 | 2005-05-03 | Hewlett-Packard Development Company, L.P. | Square-law detector based on spin injection and nanowires |
| WO2004032191A3 (en) | 2002-09-30 | 2005-06-02 | Nanosys Inc | Applications of nano-enabled large area macroelectronic substrates incorporating nanowires and nanowire composites |
| WO2005023923A3 (en) | 2003-09-04 | 2005-06-16 | Nanosys Inc | Methods of processing nanocrystals, and compositions, devices and systems including same |
| US20050145596A1 (en) | 2003-12-29 | 2005-07-07 | Metz Matthew V. | Method of fabricating multiple nanowires of uniform length from a single catalytic nanoparticle |
| US20050176228A1 (en) | 2003-12-11 | 2005-08-11 | Fonash Stephen J. | Controlled nanowire growth in permanent, integrated nano-templates and methods of fabricating sensor and transducer structures |
| US20050181195A1 (en) | 2003-04-28 | 2005-08-18 | Nanosys, Inc. | Super-hydrophobic surfaces, methods of their construction and uses therefor |
| US20050187605A1 (en) | 2002-04-11 | 2005-08-25 | Greenhalgh Skott E. | Electrospun skin capable of controlling drug release rates and method |
| US20050191774A1 (en) | 2002-10-28 | 2005-09-01 | Zhiyong Li | Photonic crystals with nanowire-based fabrication |
| US20050266662A1 (en) | 2004-05-28 | 2005-12-01 | Yi Sung S | Method of growing semiconductor nanowires with uniform cross-sectional area using chemical vapor deposition |
| US20050279274A1 (en) | 2004-04-30 | 2005-12-22 | Chunming Niu | Systems and methods for nanowire growth and manufacturing |
| US20060009003A1 (en) | 2004-07-07 | 2006-01-12 | Nanosys, Inc. | Methods for nanowire growth |
| US20060006463A1 (en) | 2004-07-09 | 2006-01-12 | Islam M S | Nanowire device with (111) vertical sidewalls and method of fabrication |
| US20060019470A1 (en) | 2004-02-06 | 2006-01-26 | Btg International Limited | Directionally controlled growth of nanowhiskers |
| US20060019472A1 (en) | 2004-04-30 | 2006-01-26 | Nanosys, Inc. | Systems and methods for nanowire growth and harvesting |
| US20060057360A1 (en) | 2003-11-26 | 2006-03-16 | Samuelson Lars I | Nanostructures formed of branched nanowhiskers and methods of producing the same |
| US7057881B2 (en) | 2004-03-18 | 2006-06-06 | Nanosys, Inc | Nanofiber surface based capacitors |
| US20060159916A1 (en) | 2003-05-05 | 2006-07-20 | Nanosys, Inc. | Nanofiber surfaces for use in enhanced surface area applications |
| US20060175601A1 (en) | 2000-08-22 | 2006-08-10 | President And Fellows Of Harvard College | Nanoscale wires and related devices |
| US7091120B2 (en) | 2003-08-04 | 2006-08-15 | Nanosys, Inc. | System and process for producing nanowire composites and electronic substrates therefrom |
| US7097820B2 (en) | 1996-08-08 | 2006-08-29 | William Marsh Rice University | Continuous fiber of single-wall carbon nanotubes |
| US7129554B2 (en) | 2000-12-11 | 2006-10-31 | President & Fellows Of Harvard College | Nanosensors |
| US7135728B2 (en) | 2002-09-30 | 2006-11-14 | Nanosys, Inc. | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US20070004225A1 (en) | 2005-06-30 | 2007-01-04 | Donghui Lu | Low-temperature catalyzed formation of segmented nanowire of dielectric material |
| US20070037365A1 (en) | 2005-08-15 | 2007-02-15 | Ranganath Tirumala R | Semiconductor nanostructures and fabricating the same |
| US20070084797A1 (en) | 2003-03-07 | 2007-04-19 | Seldon Technologies, Llc | Purification of fluids with nanomaterials |
| US7218004B2 (en) | 2005-03-11 | 2007-05-15 | Hewlett-Packard Development Company, L.P. | Fusing nanowires using in situ crystal growth |
| US20080213603A1 (en) * | 2007-03-01 | 2008-09-04 | Nobuhiko Kobayashi | Methods of forming nanostructures on metal-silicide crystallites, and resulting structures and devices |
| US20090311166A1 (en) * | 2006-05-19 | 2009-12-17 | Massachusetts Institute Of Technology | Continuous Process for the Production of Nanostructures Including Nanotubes |
| US7649192B2 (en) | 2005-03-09 | 2010-01-19 | Samsung Electronics Co., Ltd. | Nano wires and method of manufacturing the same |
-
2010
- 2010-06-28 US US12/824,485 patent/US8623288B1/en active Active
Patent Citations (143)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5332910A (en) | 1991-03-22 | 1994-07-26 | Hitachi, Ltd. | Semiconductor optical device with nanowhiskers |
| US5196396A (en) | 1991-07-16 | 1993-03-23 | The President And Fellows Of Harvard College | Method of making a superconducting fullerene composition by reacting a fullerene with an alloy containing alkali metal |
| US5505928A (en) | 1991-11-22 | 1996-04-09 | The Regents Of University Of California | Preparation of III-V semiconductor nanocrystals |
| US6048616A (en) | 1993-04-21 | 2000-04-11 | Philips Electronics N.A. Corp. | Encapsulated quantum sized doped semiconductor particles and method of manufacturing same |
| US5840435A (en) | 1993-07-15 | 1998-11-24 | President And Fellows Of Harvard College | Covalent carbon nitride material comprising C2 N and formation method |
| US5962863A (en) | 1993-09-09 | 1999-10-05 | The United States Of America As Represented By The Secretary Of The Navy | Laterally disposed nanostructures of silicon on an insulating substrate |
| US5512131A (en) | 1993-10-04 | 1996-04-30 | President And Fellows Of Harvard College | Formation of microstamped patterns on surfaces and derivative articles |
| US20030044777A1 (en) | 1993-10-28 | 2003-03-06 | Kenneth L. Beattie | Flowthrough devices for multiple discrete binding reactions |
| US5674592A (en) | 1995-05-04 | 1997-10-07 | Minnesota Mining And Manufacturing Company | Functionalized nanostructured films |
| US6190634B1 (en) | 1995-06-07 | 2001-02-20 | President And Fellows Of Harvard College | Carbide nanomaterials |
| US5690807A (en) | 1995-08-03 | 1997-11-25 | Massachusetts Institute Of Technology | Method for producing semiconductor particles |
| US6132874A (en) | 1995-08-22 | 2000-10-17 | Rhodia Chimie | Functionalized inorganic oxide hydroxylated carrier and method for preparing same |
| US6068800A (en) | 1995-09-07 | 2000-05-30 | The Penn State Research Foundation | Production of nano particles and tubes by laser liquid interaction |
| US6380103B2 (en) | 1996-01-03 | 2002-04-30 | Micron Technology, Inc. | Rapid thermal etch and rapid thermal oxidation |
| US6036774A (en) | 1996-02-26 | 2000-03-14 | President And Fellows Of Harvard College | Method of producing metal oxide nanorods |
| US5897945A (en) | 1996-02-26 | 1999-04-27 | President And Fellows Of Harvard College | Metal oxide nanorods |
| US6136156A (en) | 1996-03-01 | 2000-10-24 | Virginia Commonwealth University | Nanoparticles of silicon oxide alloys |
| US6306734B1 (en) | 1996-04-01 | 2001-10-23 | Evgeny Invievich Givargizov | Method and apparatus for growing oriented whisker arrays |
| US7097820B2 (en) | 1996-08-08 | 2006-08-29 | William Marsh Rice University | Continuous fiber of single-wall carbon nanotubes |
| US5858862A (en) | 1996-09-25 | 1999-01-12 | Sony Corporation | Process for producing quantum fine wire |
| US6130143A (en) | 1996-10-28 | 2000-10-10 | Sony Corporation | Quantum wires formed on a substrate, manufacturing method thereof, and device having quantum wires on a substrate |
| US6130142A (en) | 1996-10-28 | 2000-10-10 | Sony Corporation | Quantum wires formed on a substrate, manufacturing method thereof, and device having quantum wires on a substrate |
| US5976957A (en) | 1996-10-28 | 1999-11-02 | Sony Corporation | Method of making silicon quantum wires on a substrate |
| US5997832A (en) | 1997-03-07 | 1999-12-07 | President And Fellows Of Harvard College | Preparation of carbide nanorods |
| US6413489B1 (en) | 1997-04-15 | 2002-07-02 | Massachusetts Institute Of Technology | Synthesis of nanometer-sized particles by reverse micelle mediated techniques |
| US6207229B1 (en) | 1997-11-13 | 2001-03-27 | Massachusetts Institute Of Technology | Highly luminescent color-selective materials and method of making thereof |
| US6322901B1 (en) | 1997-11-13 | 2001-11-27 | Massachusetts Institute Of Technology | Highly luminescent color-selective nano-crystalline materials |
| US20020179434A1 (en) | 1998-08-14 | 2002-12-05 | The Board Of Trustees Of The Leland Stanford Junior University | Carbon nanotube devices |
| US6235675B1 (en) | 1998-09-22 | 2001-05-22 | Idaho Research Foundation, Inc. | Methods of forming materials containing carbon and boron, methods of forming catalysts, filaments comprising boron and carbon, and catalysts |
| US6146227A (en) | 1998-09-28 | 2000-11-14 | Xidex Corporation | Method for manufacturing carbon nanotubes as functional elements of MEMS devices |
| US6261469B1 (en) | 1998-10-13 | 2001-07-17 | Honeywell International Inc. | Three dimensionally periodic structural assemblies on nanometer and longer scales |
| US6256767B1 (en) | 1999-03-29 | 2001-07-03 | Hewlett-Packard Company | Demultiplexer for a molecular wire crossbar network (MWCN DEMUX) |
| US20010051367A1 (en) | 1999-04-14 | 2001-12-13 | Ching-Hwa Kiang | Molecular nanowires from single walled carbon nanotubes |
| US6361861B2 (en) | 1999-06-14 | 2002-03-26 | Battelle Memorial Institute | Carbon nanotubes on a substrate |
| US20020004136A1 (en) | 1999-06-14 | 2002-01-10 | Yufei Gao | Carbon nanotubes on a substrate |
| US20010033796A1 (en) | 1999-06-28 | 2001-10-25 | Unger Marc A. | Microfabricated elastomeric valve and pump sysems |
| US20020130353A1 (en) | 1999-07-02 | 2002-09-19 | Lieber Charles M. | Nanoscopic wire-based devices, arrays, and methods of their manufacture |
| US6465132B1 (en) | 1999-07-22 | 2002-10-15 | Agere Systems Guardian Corp. | Article comprising small diameter nanowires and method for making the same |
| US6790425B1 (en) | 1999-10-27 | 2004-09-14 | Wiliam Marsh Rice University | Macroscopic ordered assembly of carbon nanotubes |
| US6274007B1 (en) | 1999-11-25 | 2001-08-14 | Sceptre Electronics Limited | Methods of formation of a silicon nanostructure, a silicon quantum wire array and devices based thereon |
| US6339281B2 (en) | 2000-01-07 | 2002-01-15 | Samsung Sdi Co., Ltd. | Method for fabricating triode-structure carbon nanotube field emitter array |
| US6225198B1 (en) | 2000-02-04 | 2001-05-01 | The Regents Of The University Of California | Process for forming shaped group II-VI semiconductor nanocrystals, and product formed using process |
| US6306736B1 (en) | 2000-02-04 | 2001-10-23 | The Regents Of The University Of California | Process for forming shaped group III-V semiconductor nanocrystals, and product formed using process |
| US6294450B1 (en) | 2000-03-01 | 2001-09-25 | Hewlett-Packard Company | Nanoscale patterning for the formation of extensive wires |
| JP2001288626A (en) | 2000-04-03 | 2001-10-19 | Nissan Motor Co Ltd | Method for producing fixed body of carbon nanofiber |
| US6471761B2 (en) | 2000-04-21 | 2002-10-29 | University Of New Mexico | Prototyping of patterned functional nanostructures |
| US6586785B2 (en) | 2000-06-29 | 2003-07-01 | California Institute Of Technology | Aerosol silicon nanoparticles for use in semiconductor device fabrication |
| US6515339B2 (en) | 2000-07-18 | 2003-02-04 | Lg Electronics Inc. | Method of horizontally growing carbon nanotubes and field effect transistor using the carbon nanotubes grown by the method |
| US6803260B2 (en) | 2000-07-18 | 2004-10-12 | Lg Electronics Inc. | Method of horizontally growing carbon nanotubes and field effect transistor using the carbon nanotubes grown by the method |
| US20020014667A1 (en) | 2000-07-18 | 2002-02-07 | Shin Jin Koog | Method of horizontally growing carbon nanotubes and field effect transistor using the carbon nanotubes grown by the method |
| US20030089899A1 (en) | 2000-08-22 | 2003-05-15 | Lieber Charles M. | Nanoscale wires and related devices |
| US20020130311A1 (en) | 2000-08-22 | 2002-09-19 | Lieber Charles M. | Doped elongated semiconductors, growing such semiconductors, devices including such semiconductors and fabricating such devices |
| US7211464B2 (en) | 2000-08-22 | 2007-05-01 | President & Fellows Of Harvard College | Doped elongated semiconductors, growing such semiconductors, devices including such semiconductors and fabricating such devices |
| US20060175601A1 (en) | 2000-08-22 | 2006-08-10 | President And Fellows Of Harvard College | Nanoscale wires and related devices |
| US20030037675A1 (en) | 2000-09-05 | 2003-02-27 | Gillingham Gary R. | Air filtration arrangements having fluted media constructions and methods |
| US6495258B1 (en) | 2000-09-20 | 2002-12-17 | Auburn University | Structures with high number density of carbon nanotubes and 3-dimensional distribution |
| US20020072577A1 (en) | 2000-10-04 | 2002-06-13 | The Dow Chemical Company | Supported catalyst compositions |
| US7129554B2 (en) | 2000-12-11 | 2006-10-31 | President & Fellows Of Harvard College | Nanosensors |
| US20030071246A1 (en) | 2001-01-23 | 2003-04-17 | Grigorov Leonid N. | Quantum devices based on crystallized electron pairs and methods for their manufacture and use |
| US20020127495A1 (en) | 2001-03-12 | 2002-09-12 | Axel Scherer | Method of fabricating nanometer-scale flowchannels and trenches with self-aligned electrodes and the structures formed by the same |
| US20020158342A1 (en) | 2001-03-14 | 2002-10-31 | Mark Tuominen | Nanofabrication |
| US20030121887A1 (en) | 2001-03-22 | 2003-07-03 | Garvey James F. | Multi-component substances and processes for preparation thereof |
| US6882051B2 (en) | 2001-03-30 | 2005-04-19 | The Regents Of The University Of California | Nanowires, nanostructures and devices fabricated therefrom |
| US6996147B2 (en) | 2001-03-30 | 2006-02-07 | The Regents Of The University Of California | Methods of fabricating nanostructures and nanowires and devices fabricated therefrom |
| US20020175408A1 (en) | 2001-03-30 | 2002-11-28 | The Regents Of The University Of California | Methods of fabricating nanostructures and nanowires and devices fabricated therefrom |
| US20020172820A1 (en) | 2001-03-30 | 2002-11-21 | The Regents Of The University Of California | Methods of fabricating nanostructures and nanowires and devices fabricated therefrom |
| US20030185741A1 (en) | 2001-04-06 | 2003-10-02 | Krzysztof Matyjaszewski | Process for the preparation of nanostructured materials |
| US6656573B2 (en) | 2001-06-26 | 2003-12-02 | Hewlett-Packard Development Company, L.P. | Method to grow self-assembled epitaxial nanowires |
| US6846565B2 (en) | 2001-07-02 | 2005-01-25 | Board Of Regents, The University Of Texas System | Light-emitting nanoparticles and method of making same |
| US6808605B2 (en) | 2001-10-15 | 2004-10-26 | Korea Institute Of Science And Technology | Fabrication method of metallic nanowires |
| US6773616B1 (en) | 2001-11-13 | 2004-08-10 | Hewlett-Packard Development Company, L.P. | Formation of nanoscale wires |
| US20040005258A1 (en) | 2001-12-12 | 2004-01-08 | Fonash Stephen J. | Chemical reactor templates: sacrificial layer fabrication and template use |
| US20030200521A1 (en) | 2002-01-18 | 2003-10-23 | California Institute Of Technology | Array-based architecture for molecular electronics |
| US20030232721A1 (en) | 2002-01-31 | 2003-12-18 | Hydrocarbon Technologies Inc. | Supported noble metal nanometer catalyst particles containing controlled (111) crystal face exposure |
| US20030184357A1 (en) | 2002-02-19 | 2003-10-02 | Commissariat A L'energie Atomique | Cathode structure with emissive layer formed on a resistive layer |
| US6515325B1 (en) | 2002-03-06 | 2003-02-04 | Micron Technology, Inc. | Nanotube semiconductor devices and methods for making the same |
| US7081385B2 (en) | 2002-03-06 | 2006-07-25 | Micron Technology, Inc. | Nanotube semiconductor devices and methods for making the same |
| US20040026684A1 (en) | 2002-04-02 | 2004-02-12 | Nanosys, Inc. | Nanowire heterostructures for encoding information |
| US6872645B2 (en) | 2002-04-02 | 2005-03-29 | Nanosys, Inc. | Methods of positioning and/or orienting nanostructures |
| US20040005723A1 (en) | 2002-04-02 | 2004-01-08 | Nanosys, Inc. | Methods of making, positioning and orienting nanostructures, nanostructure arrays and nanostructure devices |
| US6962823B2 (en) | 2002-04-02 | 2005-11-08 | Nanosys, Inc. | Methods of making, positioning and orienting nanostructures, nanostructure arrays and nanostructure devices |
| US20050230356A1 (en) | 2002-04-02 | 2005-10-20 | Nanosys, Inc. | Methods of making, positioning and orienting nanostructures, nanostructure arrays and nanostructure devices |
| US20030186522A1 (en) | 2002-04-02 | 2003-10-02 | Nanosys, Inc. | Methods of positioning and/or orienting nanostructures |
| US6831017B1 (en) | 2002-04-05 | 2004-12-14 | Integrated Nanosystems, Inc. | Catalyst patterning for nanowire devices |
| US20030189202A1 (en) | 2002-04-05 | 2003-10-09 | Jun Li | Nanowire devices and methods of fabrication |
| US20030195611A1 (en) | 2002-04-11 | 2003-10-16 | Greenhalgh Skott E. | Covering and method using electrospinning of very small fibers |
| US20050187605A1 (en) | 2002-04-11 | 2005-08-25 | Greenhalgh Skott E. | Electrospun skin capable of controlling drug release rates and method |
| US20030222019A1 (en) | 2002-05-28 | 2003-12-04 | Battelle Memorial Institute | Electrostatic deposition of particles generated from rapid expansion of supercritical fluid solutions |
| US20040213307A1 (en) | 2002-07-19 | 2004-10-28 | President And Fellows Of Harvard College | Nanoscale coherent optical components |
| US20040095658A1 (en) | 2002-09-05 | 2004-05-20 | Nanosys, Inc. | Nanocomposites |
| US6878871B2 (en) | 2002-09-05 | 2005-04-12 | Nanosys, Inc. | Nanostructure and nanocomposite based compositions and photovoltaic devices |
| US20040118448A1 (en) | 2002-09-05 | 2004-06-24 | Nanosys, Inc. | Nanostructure and nanocomposite based compositions and photovoltaic devices |
| US20040146560A1 (en) | 2002-09-05 | 2004-07-29 | Nanosys, Inc. | Oriented nanostructures and methods of preparing |
| US20040061422A1 (en) | 2002-09-26 | 2004-04-01 | International Business Machines Corporation | System and method for molecular optical emission |
| US20040063839A1 (en) | 2002-09-26 | 2004-04-01 | Canon Kabushiki Kaisha | Method of producing electron emitting device using carbon fiber, electron source and image forming apparatus, and ink for producing carbon fiber |
| WO2004032193A3 (en) | 2002-09-30 | 2005-01-27 | Nanosys Inc | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US20040135951A1 (en) | 2002-09-30 | 2004-07-15 | Dave Stumbo | Integrated displays using nanowire transistors |
| US20050079659A1 (en) | 2002-09-30 | 2005-04-14 | Nanosys, Inc. | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US7064372B2 (en) | 2002-09-30 | 2006-06-20 | Nanosys, Inc. | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US7051945B2 (en) | 2002-09-30 | 2006-05-30 | Nanosys, Inc | Applications of nano-enabled large area macroelectronic substrates incorporating nanowires and nanowire composites |
| US7067867B2 (en) | 2002-09-30 | 2006-06-27 | Nanosys, Inc. | Large-area nonenabled macroelectronic substrates and uses therefor |
| WO2004032191A3 (en) | 2002-09-30 | 2005-06-02 | Nanosys Inc | Applications of nano-enabled large area macroelectronic substrates incorporating nanowires and nanowire composites |
| US20040112964A1 (en) | 2002-09-30 | 2004-06-17 | Nanosys, Inc. | Applications of nano-enabled large area macroelectronic substrates incorporating nanowires and nanowire composites |
| US7135728B2 (en) | 2002-09-30 | 2006-11-14 | Nanosys, Inc. | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US7233041B2 (en) | 2002-09-30 | 2007-06-19 | Nanosys, Inc. | Large-area nanoenabled macroelectronic substrates and uses therefor |
| US20050191774A1 (en) | 2002-10-28 | 2005-09-01 | Zhiyong Li | Photonic crystals with nanowire-based fabrication |
| US20040147098A1 (en) | 2002-11-22 | 2004-07-29 | Frederic Mazen | Method for forming, by CVD, nanostructures of semi-conductor material of homogeneous and controlled size on dielectric material |
| US20040121681A1 (en) | 2002-12-23 | 2004-06-24 | Kimberly-Clark Worldwide, Inc. | Absorbent articles containing an activated carbon substrate |
| US20070084797A1 (en) | 2003-03-07 | 2007-04-19 | Seldon Technologies, Llc | Purification of fluids with nanomaterials |
| US20050011431A1 (en) | 2003-04-04 | 2005-01-20 | Btg International Limited | Precisely positioned nanowhiskers and nanowhisker arrays and method for preparing them |
| US20040206448A1 (en) | 2003-04-17 | 2004-10-21 | Nanosys, Inc. | Structures, systems and methods for joining articles and materials and uses therefor |
| US20050038498A1 (en) | 2003-04-17 | 2005-02-17 | Nanosys, Inc. | Medical device applications of nanostructured surfaces |
| US7056409B2 (en) | 2003-04-17 | 2006-06-06 | Nanosys, Inc. | Structures, systems and methods for joining articles and materials and uses therefor |
| US20050181195A1 (en) | 2003-04-28 | 2005-08-18 | Nanosys, Inc. | Super-hydrophobic surfaces, methods of their construction and uses therefor |
| US20060159916A1 (en) | 2003-05-05 | 2006-07-20 | Nanosys, Inc. | Nanofiber surfaces for use in enhanced surface area applications |
| US20050029678A1 (en) | 2003-07-08 | 2005-02-10 | University Of Texas System, Board Of Regents | Growth of single crystal nanowires |
| US20050026526A1 (en) | 2003-07-30 | 2005-02-03 | Verdegan Barry M. | High performance filter media with internal nanofiber structure and manufacturing methodology |
| US6888208B2 (en) | 2003-07-30 | 2005-05-03 | Hewlett-Packard Development Company, L.P. | Square-law detector based on spin injection and nanowires |
| US7091120B2 (en) | 2003-08-04 | 2006-08-15 | Nanosys, Inc. | System and process for producing nanowire composites and electronic substrates therefrom |
| US20050062033A1 (en) | 2003-08-08 | 2005-03-24 | Canon Kabushiki Kaisha | Structure and method for production of the same |
| WO2005023923A3 (en) | 2003-09-04 | 2005-06-16 | Nanosys Inc | Methods of processing nanocrystals, and compositions, devices and systems including same |
| US7067328B2 (en) | 2003-09-25 | 2006-06-27 | Nanosys, Inc. | Methods, devices and compositions for depositing and orienting nanostructures |
| US20050066883A1 (en) | 2003-09-25 | 2005-03-31 | Nanosys, Inc. | Methods, devices and compositions for depositing and orienting nanostructures |
| US20050089467A1 (en) | 2003-10-22 | 2005-04-28 | International Business Machines Corporation | Control of carbon nanotube diameter using CVD or PECVD growth |
| US20060057360A1 (en) | 2003-11-26 | 2006-03-16 | Samuelson Lars I | Nanostructures formed of branched nanowhiskers and methods of producing the same |
| US20050176228A1 (en) | 2003-12-11 | 2005-08-11 | Fonash Stephen J. | Controlled nanowire growth in permanent, integrated nano-templates and methods of fabricating sensor and transducer structures |
| US20050145596A1 (en) | 2003-12-29 | 2005-07-07 | Metz Matthew V. | Method of fabricating multiple nanowires of uniform length from a single catalytic nanoparticle |
| US20060019470A1 (en) | 2004-02-06 | 2006-01-26 | Btg International Limited | Directionally controlled growth of nanowhiskers |
| US7057881B2 (en) | 2004-03-18 | 2006-06-06 | Nanosys, Inc | Nanofiber surface based capacitors |
| US20050279274A1 (en) | 2004-04-30 | 2005-12-22 | Chunming Niu | Systems and methods for nanowire growth and manufacturing |
| US7105428B2 (en) | 2004-04-30 | 2006-09-12 | Nanosys, Inc. | Systems and methods for nanowire growth and harvesting |
| US20060255481A1 (en) | 2004-04-30 | 2006-11-16 | Nanosys, Inc. | Systems and methods for nanowire growth and harvesting |
| US20060019472A1 (en) | 2004-04-30 | 2006-01-26 | Nanosys, Inc. | Systems and methods for nanowire growth and harvesting |
| US20050266662A1 (en) | 2004-05-28 | 2005-12-01 | Yi Sung S | Method of growing semiconductor nanowires with uniform cross-sectional area using chemical vapor deposition |
| US20060009003A1 (en) | 2004-07-07 | 2006-01-12 | Nanosys, Inc. | Methods for nanowire growth |
| US20060008942A1 (en) | 2004-07-07 | 2006-01-12 | Nanosys, Inc. | Systems and methods for harvesting and integrating nanowires |
| US20060006463A1 (en) | 2004-07-09 | 2006-01-12 | Islam M S | Nanowire device with (111) vertical sidewalls and method of fabrication |
| US7649192B2 (en) | 2005-03-09 | 2010-01-19 | Samsung Electronics Co., Ltd. | Nano wires and method of manufacturing the same |
| US7218004B2 (en) | 2005-03-11 | 2007-05-15 | Hewlett-Packard Development Company, L.P. | Fusing nanowires using in situ crystal growth |
| US20070004225A1 (en) | 2005-06-30 | 2007-01-04 | Donghui Lu | Low-temperature catalyzed formation of segmented nanowire of dielectric material |
| US20070037365A1 (en) | 2005-08-15 | 2007-02-15 | Ranganath Tirumala R | Semiconductor nanostructures and fabricating the same |
| US20090311166A1 (en) * | 2006-05-19 | 2009-12-17 | Massachusetts Institute Of Technology | Continuous Process for the Production of Nanostructures Including Nanotubes |
| US20080213603A1 (en) * | 2007-03-01 | 2008-09-04 | Nobuhiko Kobayashi | Methods of forming nanostructures on metal-silicide crystallites, and resulting structures and devices |
Non-Patent Citations (34)
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090255222A1 (en) * | 2007-07-10 | 2009-10-15 | Raul Cortez | Methods and apparatus for the in situ collection of nucleated particles |
| US20130092525A1 (en) * | 2007-07-10 | 2013-04-18 | Innovalight, Inc. | Concentric flow-through plasma reactor and methods therefor |
| US8968438B2 (en) | 2007-07-10 | 2015-03-03 | Innovalight, Inc. | Methods and apparatus for the in situ collection of nucleated particles |
| US10243207B2 (en) | 2011-07-26 | 2019-03-26 | Oned Material Llc | Nanostructured battery active materials and methods of producing same |
| US11616225B2 (en) | 2011-07-26 | 2023-03-28 | Oned Material, Inc. | Nanostructured battery active materials and methods of producing same |
| US9812699B2 (en) | 2011-10-05 | 2017-11-07 | Oned Material Llc | Silicon nanostructure active materials for lithium ion batteries and processes, compositions, components and devices related thereto |
| US10355266B2 (en) | 2011-10-05 | 2019-07-16 | Oned Material Llc | Silicon nanostructure active materials for lithium ion batteries and processes, compositions, components and devices related thereto |
| US10804525B2 (en) | 2011-10-05 | 2020-10-13 | Oned Material Inc. | Silicon nanostructure active materials for lithium ion batteries and processes, compositions, components, and devices related thereto |
| US10862114B2 (en) | 2016-07-15 | 2020-12-08 | Oned Material Llc | Manufacturing apparatus and method for making silicon nanowires on carbon based powders for use in batteries |
| US11728477B2 (en) | 2016-07-15 | 2023-08-15 | Oned Material, Inc. | Manufacturing apparatus and method for making silicon nanowires on carbon based powders for use in batteries |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8623288B1 (en) | Apparatus and methods for high density nanowire growth | |
| US7985454B2 (en) | Systems and methods for nanowire growth and manufacturing | |
| US20060009003A1 (en) | Methods for nanowire growth | |
| KR101110409B1 (en) | Method of manufacturing carbon nanostructure | |
| WO2010092786A1 (en) | Base for producing oriented carbon nanotube aggregate, and method for producing oriented carbon nanotube aggregate | |
| JP2012519643A (en) | System and method for surface treatment and barrier coating of in situ CNT growth fibers | |
| EP3394316B1 (en) | Fluidized bed reactor adapted for the production of biphased systems and method for a controlled-deposition of particles | |
| JPWO2007116434A1 (en) | Method for producing carbon nanotube | |
| Kitsomboonloha et al. | Selective growth of zinc oxide nanorods on inkjet printed seed patterns | |
| Kharissova et al. | Less-common nanostructures in the forms of vegetation | |
| KR101268270B1 (en) | Hydrophobic layer having nanoparticles and method for fabricating the same | |
| KR100583610B1 (en) | Febrication method of transition metal oxide/carbon nanotube composite | |
| EP2727127A1 (en) | Device comprising nanostructures and method of manufacturing thereof | |
| US8859449B2 (en) | Fine-particle structure/substrate composite member and method for producing same | |
| US11041236B2 (en) | Method for direct patterned growth of atomic layer metal dichalcogenides with pre-defined width | |
| JP7167053B2 (en) | Carbon nanostructure manufacturing method and carbon nanostructure manufacturing apparatus | |
| US9056777B2 (en) | Bent carbon nanotubes and methods of production | |
| TWI780298B (en) | A photocatalytic structure and method for making the same | |
| Ayyub | Aligned nanorod arrays: additive and emergent properties | |
| CN111489897B (en) | Photocatalytic structure and preparation method thereof | |
| KR101122129B1 (en) | Method for preparing Si/SiOx core/shell nanowire by using Si-rich oxides | |
| WO2007145080A1 (en) | Carbon nanotube network composite material and method for manufacturing same | |
| Hwang et al. | Deposition Behavior of Charged Nanoparticles | |
| KR20160096479A (en) | Nano structures, and method for manufacturing the same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: NANOSYS, INC., CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:DUBROW, ROBERT S.;CASILLAS, CARLOS;SIGNING DATES FROM 20100723 TO 20100726;REEL/FRAME:024782/0499 |
|
| AS | Assignment |
Owner name: NANOSYS, INC., CALIFORNIA Free format text: SECURITY AGREEMENT;ASSIGNOR:PRVP HOLDINGS, LLC;REEL/FRAME:030285/0829 Effective date: 20121221 |
|
| AS | Assignment |
Owner name: NANOSYS, INC., CALIFORNIA Free format text: RELEASE BY SECURED PARTY;ASSIGNOR:NANOSYS, INC.;REEL/FRAME:030599/0907 Effective date: 20130611 |
|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| AS | Assignment |
Owner name: ONED MATERIAL LLC, CALIFORNIA Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:NANOSYS, INC.;REEL/FRAME:032264/0148 Effective date: 20131231 |
|
| AS | Assignment |
Owner name: MPEG LA, L.L.C., COLORADO Free format text: SECURITY INTEREST;ASSIGNOR:ONED MATERIAL LLC;REEL/FRAME:032447/0937 Effective date: 20131231 |
|
| AS | Assignment |
Owner name: MPEG LA, L.L.C., MARYLAND Free format text: SECURITY INTEREST;ASSIGNOR:ONED MATERIAL LLC;REEL/FRAME:034171/0227 Effective date: 20140801 |
|
| AS | Assignment |
Owner name: ONED MATERIAL LLC, CALIFORNIA Free format text: RELEASE BY SECURED PARTY;ASSIGNOR:MPEG LA, L.L.C;REEL/FRAME:040373/0380 Effective date: 20161005 Owner name: ONED MATERIAL LLC, CALIFORNIA Free format text: RELEASE BY SECURED PARTY;ASSIGNOR:MPEG LA, L.L.C;REEL/FRAME:040373/0484 Effective date: 20161005 |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| AS | Assignment |
Owner name: ONED MATERIAL, INC., CALIFORNIA Free format text: CHANGE OF NAME;ASSIGNOR:ONED MATERIAL LLC;REEL/FRAME:053375/0462 Effective date: 20191231 |
|
| MAFP | Maintenance fee payment |
Free format text: PAYMENT OF MAINTENANCE FEE, 8TH YEAR, LARGE ENTITY (ORIGINAL EVENT CODE: M1552); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Year of fee payment: 8 |